Author: [AUTHOR] Published on 2/15/2021 1:03:00 PM
FDA Approves CABENUVA (Cabotegravir and Rilpivirine) and VOCABRIA (Cabotegravir) for HIV in Patients who are Virologically Suppressed on a Stable Antiretroviral Regimen
On January 21, 2021, the US Food and Drug Administration (FDA) approved CABENUVA (cabotegravir extended release injectable suspension; rilpivirine extended release injectable suspension) and VOCABRIA (cabotegravir tablets). CABENUVA is indicated as a complete regimen for the treatment of HIV-1 infection in adults to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA < 50 copies/mL) on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine. VOCABRIA is used in combination with approved EDURANT (rilpivirine) tablets.
A recommended oral lead-in of VOCABRIA should be used prior to the initiation of CABENUVA to assess the tolerability of cabotegravir and rilpivirine.
- The recommended dosage of VOCABRIA is 30 mg in combination with EDURANT 25 mg. Administer VOCABRIA and EDURANT orally once daily with a meal for approximately 1 month (at least 28 days).
CABENUVA must be administered by a healthcare professional. CABENUVA requires an initiation injection followed by monthly continuation injections. A complete dose of CABENUVA requires 2 intramuscular (IM) injections: one cabotegravir injection and one rilpivirine injection. Administer cabotegravir and rilpivirine at separate gluteal injection sites (on opposite sides or 2 cm apart) during the same visit.
- Initial injections on the last day of oral lead-in: Cabotegravir 600 mg and rilpivirine 900 mg
- Monthly continuation injections: Cabotegravir 400 mg and rilpivirine 600 mg
Additional information regarding dosage and administration as well as warnings and precautions about hypersensitivity reactions, post-injection reactions, hepatotoxicity, depressive disorders, risk of adverse reactions or loss of virologic response due to drug interactions, long-acting properties and potential associated risks with CABENUVA can be found in the full prescribing information linked below.
Mechanism of Action (MOA), Pharmacokinetics (PK), and Pharmacodynamics (PD)
MOA: Cabotegravir is an HIV-1 integrase strand transfer inhibitor (INSTI) and rilpivirine is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI).
PK: The PK properties of the components of CABENUVA are provided in Table 1 below.
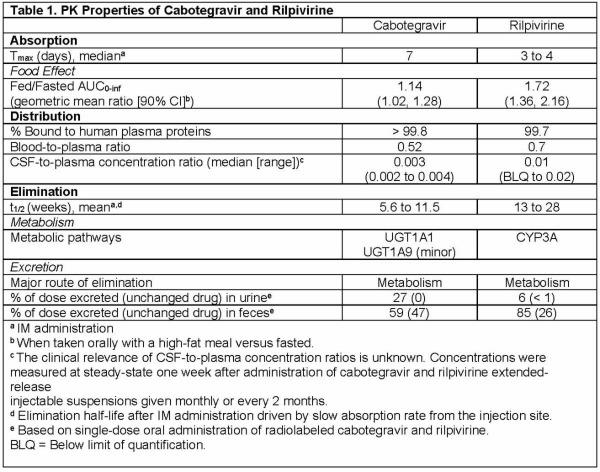
The multiple-dose PK exposure parameters of cabotegravir and rilpivirine are provided in Table 2.
 Cardiac Electrophysiology
Cardiac Electrophysiology: Due to prolongation of the QTc interval at supratherapeutic exposures of rilpivirine, CABENUVA should be used with caution in combination with drugs with a known risk of Torsade de Pointes.
Drug Interactions
Co-administration of CABENUVA is contraindicated with inducers of uridine diphosphate (UDP)-glucuronosyl transferase (UGT)1A1 and/or cytochrome P450 (CYP)3A enzymes, which cause significant decreases in cabotegravir and/or rilpivirine plasma concentrations, and may result in loss of virologic response.
- Anticonvulsants: Carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- Antimycobacterials: Rifabutin, rifampin, rifapentine
- Glucocorticoid (systemic): Dexamethasone (more than a single-dose treatment)
- Herbal product: St. John’s wort
Other Antiretroviral Medications for the Treatment of HIV-1 Infection: Because CABENUVA is a complete regimen, co-administration with other antiretrovirals is not recommended. Following discontinuation of CABENUVA, residual concentrations of cabotegravir and rilpivirine may remain in the systemic circulation of patients for prolonged periods (up to 12 months or longer).
Macrolide or Ketolide Antibiotics: Where possible, consider alternatives, such as azithromycin, which increases rilpivirine concentrations less than other macrolides since macrolides are expected to increase concentrations of rilpivirine and are associated with a risk of Torsade de Pointes.
Narcotic Analgesic: No dose adjustment of methadone is required when co-administered with CABENUVA. However, clinical monitoring is recommended as methadone maintenance therapy may need to be adjusted in some patients.
Refer to the prescribing information for VOCABRIA and EDURANT for additional drug interaction information related to oral cabotegravir and oral rilpivirine, respectively.
Use in Specific Populations
No clinically significant differences in the PK of cabotegravir or rilpivirine were observed based on age, sex, race/ethnicity, body mass index, or UGT1A1 polymorphisms. The pharmacokinetics of cabotegravir (oral or injectable) or injectable rilpivirine have not been studied in pediatric patients and data are limited in subjects aged 65 years or older.
No clinically significant differences in the PK of cabotegravir are expected in mild to moderate (Child-Pugh A or B) hepatic impairment. The effect of severe hepatic impairment (Child-Pugh C) and hepatitis B and C virus co-infection on the PK of cabotegravir is unknown.
Renal Impairment: No dosage adjustment of CABENUVA is necessary for patients with mild (CLcr 60-89 mL/min) or moderate renal impairment (CLcr 30-59 mL/min). Increased monitoring for adverse effects is recommended in patients with severe renal impairment (CLcr 15-29 mL/min) or end-stage renal disease (CLcr < 15 mL/min). In patients with end-stage renal disease not on dialysis, effects on the PK of cabotegravir or rilpivirine are unknown. Dialysis is not expected to alter exposures of cabotegravir or rilpivirine.
Efficacy and Safety
Efficacy of CABENUVA was demonstrated in two Phase 3 randomized, multicenter, active-controlled, parallel-arm, open-label, non-inferiority trials. The primary endpoint of these trials was the proportion of subjects with plasma HIV-1 RNA ≥ 50 copies/mL at Week 48. Additional information regarding the efficacy trials can be found in the full prescribing information linked below.
The most common adverse reactions (Grades 1 to 4) observed in ≥ 2% of subjects receiving CABENUVA were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash.
Full prescribing information for CABENUVA is available at
https://go.usa.gov/xA6Kz.
Full prescribing information for VOCABRIA is available at
https://go.usa.gov/xsrtK.
Full prescribing information for EDURANT is available at
https://go.usa.gov/xAMmh.
Visit Drugs@FDA at
http://go.usa.gov/cMsjT for prescribing and patient information, approval letters, reviews and other information for FDA-approved drug products, which are often available shortly following approval.
A non-comprehensive list of examples of clinical substrates, inhibitors and inducers for metabolic and transporter system related interactions may be found at
https://go.usa.gov/xXY9C.
Healthcare professionals should report all serious adverse events suspected to be associated with the use of any medicine and device to FDA's MedWatch Reporting System by completing a form online at
http://www.fda.gov/medwatch/report.htm, by faxing (1-800-FDA-0178), or by mailing the postage-paid address form provided online, or by telephone (1-800-FDA-1088).
The Office of Clinical Pharmacology (OCP) is pleased to offer the e-mail subscription service Clinical Pharmacology Corner. This is a free service from FDA to provide occasional updates from OCP regarding newly approved therapies, new regulatory and scholarly publications, upcoming events and other items of interest. Subscribe today at
http://go.fda.gov/subscriptionmanagement (note: this link does not work with Internet Explorer) and select Clinical Pharmacology Corner under Drugs.
We always welcome your thoughts regarding the format, content, and utility of this communication. Comments may be sent via email to
ocp@fda.hhs.gov.
This communication was prepared by Office of Clinical Pharmacology, Office of Translational Sciences, CDER, FDA.
