Author: Mark Dresser, PhD on April 18, 2016 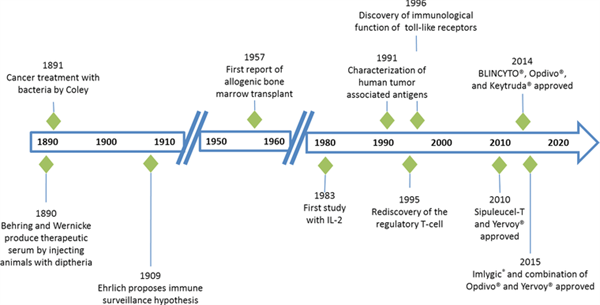
There has been unprecedented progress in the treatment of cancer with the recent advances in the discovery and development of cancer immunotherapies, a relatively new class of agents that activate a patient’s immune system to recognize and eliminate tumors. For example, overall survival benefits have been reported for a number of these therapeutics in tumor types that were previously refractory to all other interventions1-3. Despite this encouraging progress, many patients fail to respond to cancer immunotherapies and there remain many unresolved questions such as how best to select patients who will respond to treatment and how to optimally dose and combine therapies for maximum therapeutic benefit. A recent review article published in Clinical and Translational Science (CTS) by Morrissey et al. provides a comprehensive overview of the history, current status, challenges and future opportunities for immunotherapies. This review will be a valuable resource to researchers, clinicians, drug developers, and regulators currently working in the cancer immunology field as well as to individuals who are new to this area of therapeutic research and development.
In the first part of their review, Morrissey et al. provide an overview of the history of cancer immunotherapy and a comprehensive summary of the approved therapies. Clinical development and trial design considerations are covered in the second part, including helpful strategies to identify efficacious doses and schedules for this class of therapeutics, to select patients, and to incorporate biomarkers into clinical trials. In the third and fourth sections of the review, the authors address key issues related to clinical pharmacology considerations and safety and tolerability considerations for cancer immunotherapies. Regulatory considerations are discussed in the fifth and final section of the review, including the pros and cons and limitations of existing surrogate end-points to predict long-term survival and the need to further develop and characterize end points that take the unique tumor response kinetics of these therapeutics into account.
Morrissey et al. conclude their review suggesting that “combination immunotherapy is the future of cancer treat” and that “the success of combination immunotherapy is dependent on our ability to manage development hurdles including (i) the design of the right preclinical experiments and the translation of those experiments into the clinic, (ii) optimization of the dose and schedule of the combination regimen, and (iii) management of the overlapping toxicities that can be expected based on the mechanism of action of immunotherapies. The application of quantitative clinical pharmacology approaches in the translational space and throughout clinical development may help to address these challenges.” CTS welcomes high quality, scientifically sound manuscripts focused on the translational science and translational medicine aspects of cancer immunotherapy.
References:
1. Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711-23.
2. Borghaei H, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015 Oct 22;373(17):1627-39.
3. Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015 May 21;372(21):2018-28.
Image by Morrissey, et al. Clin. Trans. Sci., doi: 10.1111/cts.12391, is licensed under CC BY-NC-ND 4.0. ©2016 The authors.

The comment feature is locked by administrator.