Author: John A. Wagner, MD, PhD on July 03, 2018 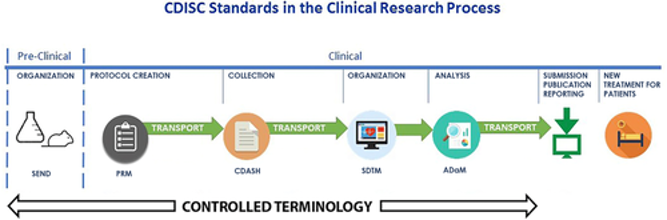
“Without standards, there can be no improvement.”
– Taiichi Ohno
Have you traveled internationally recently? If you have, I’m sure you’ve had to lug around an electrical adaptor or two to recharge your devices. Why? Because the world does not have one standard for electrical plugs. Can you imagine how convenient it would be if there was one worldwide standard? Not only is the lack of standards a pain, it impedes progress. Ohno-san was a Japanese industrial engineer and businessman, considered to be the father of the Toyota Production System. His approach to quality and standards drove progress in automobile manufacturing for decades. Standards – or the lack thereof – is also critical for biomedical research, and, in particular, clinical data sharing. If electrical plugs are a problem, it’s easy to imagine how we collect, understand, use, and exchange data could vary widely among investigators around the globe or across the hall.
The commentary from Hudson et al., “Global Standards to Expedite Learning From Medical Research Data,” elaborates on standards in clinical data sharing, and how standardization can drive common purposes in clinical research forward. The authors summarize work ongoing by Coalition for Advancing Standards and Therapies (CFAST), a Critical Path Institute consortium. The value of standardization has clearly demonstrated for data in dossiers submitted to regulators in support of approval of new medicines. But transparent data sharing continues to be an important goal for stakeholders from academics, to regulators, to biopharmaceutical researchers. Lack of consistent and widely adopted standards have impaired such data sharing. Hudson et al. argue that such standardization, although difficult, drives important scientific, medical and economic benefits.
Although standardization sounds like a mundane problem, the lack of standards stands in the way of progress on projects ranging from the Cancer Moonshot to Alzheimer’s disease. Think of the biomedical research equivalent to the Toyota Production System. The implications are profound and far-reaching. Hudson et al. point out that the tools and the policies to apply those standardization tools will shape – and drive – our data‐sharing future. Investment is well worth the effort, since standardization can vastly benefit biomedical research. CTS welcomes your contribution to standardization whether related to clinical data sharing, biomarkers, or drug development.
Image by Hudson, et al. Clin. Trans. Sci., doi: 10.1111/cts.12556, is licensed under CC BY-NC 4.0. ©2018 The authors.

The comment feature is locked by administrator.