Author: Deanna L. Kroetz, PhD on March 17, 2020 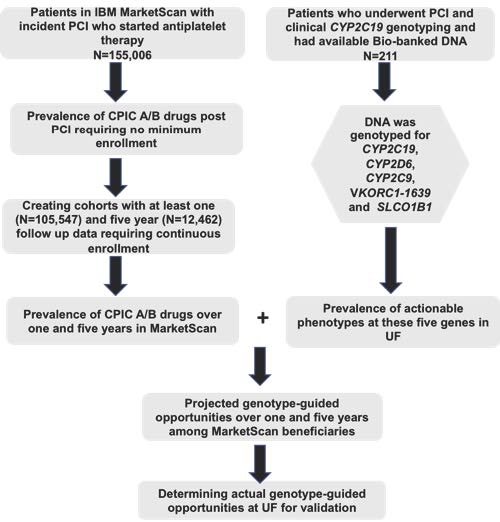
Pharmacogene genotyping is almost always reactionary and triggered by the prescribing of an actionable drug. A barrier to pharmacogenetic clinical implementation in the context of reactionary genotyping are delays in the turnaround of genetic data to prescribers. Many experts have argued that preemptive genotyping would eliminate these delays, but this seemingly simple fix is hindered by our current reimbursement system.
A recent original research article in Clinical and Translational Science by El Rouby et al. provides compelling data to suggest that preemptive genotyping of a panel of pharmacogenes will not only accelerate clinical implementation but will also be cost-effective. Pharmacogenetic testing is often used in the setting of percutaneous coronary intervention (PCI), making this an excellent test case to examine the incidence of future drug treatments that might benefit from genotype-guided dosing. Data was extracted from the IBM MarketScan beneficiary database for more than 150,000 patients who underwent percutaneous coronary intervention (PCI) and started antiplatelet therapy between 2008 and 2015. Further analysis demonstrated that 36% of these patients were prescribed at least one additional drug deemed actionable by the Clinical Pharmacogenetics Implementation Consortium within a one-year period following PCI and antiplatelet therapy, and 25% of them were prescribed three or more actionable drugs within five years. Examination of actionable phenotypes in a University of Florida cohort with panel-based pharmacogene genotyping was consistent with the projections based on the MarketScan data. The question now is whether such data will compel payers to reconsider preemptive pharmacogene panel-based testing. Perhaps the financial argument of getting multiple actionable genotypes for the price of a single test will finally be enough to broaden the impact of clinical implementation of pharmacogenetics beyond a handful of academic medical centers.
This article highlights the power of analysis of large claims databases in support of the implementation of pharmacogenetic findings. The Editorial Team of Clinical and Translational Science welcomes original pharmacogenetic contributions spanning the entire spectrum from discovery to implementation.
Image by El Rouby et al. Clin. Trans. Sci https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.12729. Published 2019. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.

The comment feature is locked by administrator.