Author: John A. Wagner, MD, PhD on October 26, 2021 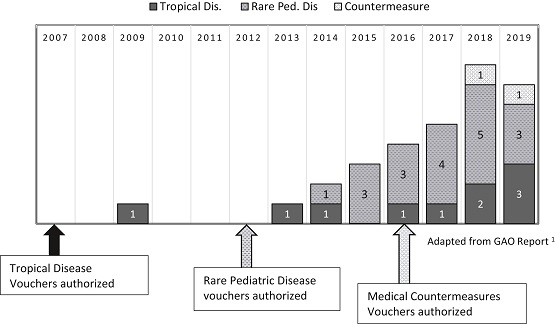
The US Food and Drug Administration (FDA) priority review voucher (PRV) program, which provides incentives for drug development in areas of the greatest unmet need and where traditional incentives do not appear to be working, has been controversial from its onset. Neglected tropical diseases was the first area for PRV incentives, passed into law in 2007. Subsequently, PRVs were expanded to rare pediatric diseases and medical countermeasures. In 2016, Congress asked the Government Accounting Office (GAO) to conduct a “study addressing the effectiveness and overall impact of the…priority review voucher programs.” Recently, Meyer published a Commentary in Clinical and Translational Science (CTS) summarizing that report. It turns out that the GAO report suggests that PRV programs have had “little or no effect” on incentivizing drug development in the designated high medical need areas. The report’s findings call into question the effectiveness of the PRV program in general and should be considered by policy makers as they consider the value of PRV incentives drug development in the future.
PRVs remain in the news. In September, FDA issued a Federal Register notice, Fee Rate for Using a Rare Pediatric Disease Priority Review Voucher in Fiscal Year 2022. This notice defines the rare pediatric disease priority review fee rate for the coming financial year. Pharma and biotech news have picked this story up, since the fee decreases again as the cost of a priority assessment inches closer to the cost of a standard assessment. It appears that regulators may also be commenting on the value of PRVs.
The CTS editorial team invites you to submit your translational research to CTS. Whether your work highlights original translational medicine research that helps bridge laboratory discoveries with the diagnosis and treatment of human disease or is an insightful commentary on regulatory policy, please consider CTS for your next contribution.

The comment feature is locked by administrator.