Author: John A. Wagner, MD, PhD on March 01, 2022 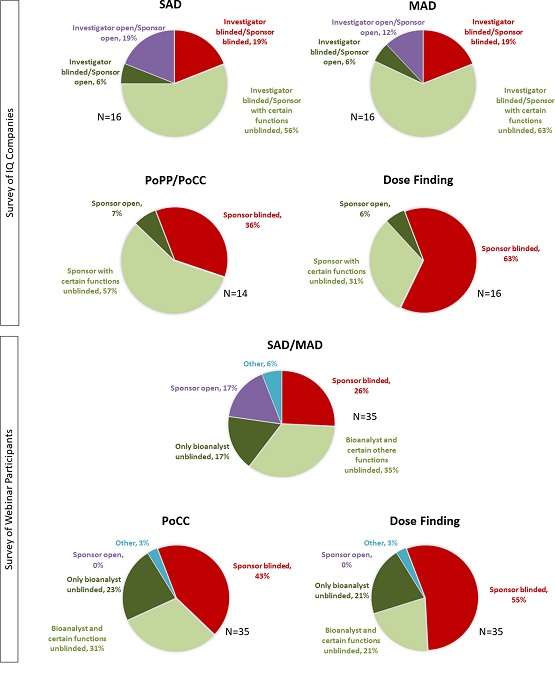
To blind or not to blind – that is the question asked my numerous scientists across multiple stakeholders, particularly with regard to early clinical phase 1 studies. Many authors have opined for or against blinding in various phase 1 settings, but an IQ Consortium working group recently completed a survey of biopharmaceutical companies to delve into industry blinding practices. A healthy debate is good for the fields of translational science and drug development. Arguments against blinding include added expense, complexity, and possibly duration (e.g., formulation timelines). However, there are many strong reasons for blinding early clinical phase 1 studies, including robust decision making, interpretation and context for safety and tolerability signals, conduct of rigorously controlled experiments, ability to interpret the many surprises that occur in clinical research, and the fact that for novel biomarker development, blinded experimental provide controls with important data for which baseline values are not adequate.
Haertter et al. recently published the results of the IQ survey in Clinical and Translational Science (CTS), providing solid, timely data for discussions on blinding as well as the perceived risk of bias. On one hand, 19% of the sponsors conducted single ascending dose/multiple ascending dose (SAD/MAD) studies with all sponsor functions completely blinded. On the other hand, 19% of sponsors conducted SAD studies and 12% of sponsors conducted MAD studies with all sponsor functions and the investigator unblinded (single blind). Fifty six percent of SAD and 63% for MAD studies were conducted with selected sponsor functions unblinded during trial conduct. Thus, the overwhelming majority of sponsors ran blinded SAD/MAD phase 1 studies. The Haertter et al. survey also extends the scope to exploratory, early clinical patient trials and, again, the majority of sponsors engage in blinded designs. Based on these results, the IQ working group developed strategic considerations for first-in-human healthy volunteer trials and early clinical, exploratory trials in patients.
The CTS Editorial Team invites submissions of translational research on how to perform drug development and academic research. Please consider submitting to CTS.

The comment feature is locked by administrator.