Author: Tal Burt, MD on April 21, 2022 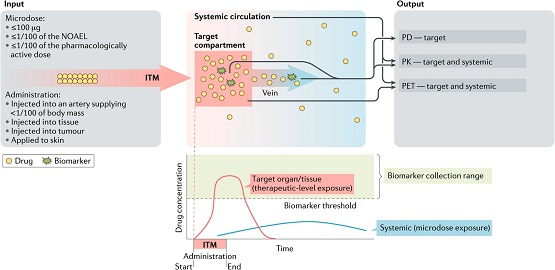
Among the many efforts to improve productivity in drug development are Phase-0 approaches that use sub-therapeutic exposures in First-in-Human (FIH) studies. The low exposures and the implied safety allow reduced regulatory packages and entry into human testing earlier and with fewer resources than required by traditional approaches. The quick and effective ‘read’ allows for a human-based selection among pre-clinical candidates, some of which may otherwise be deselected based on in-vivo and/or in-vitro data alone. A recent paper by Burt et al. in Clinical and Translational Science (CTS) details the operational aspects of Phase-0 and is a companion to another recent Nature Reviews Drug Discovery paper on Phase-0 validity, methodology, and applications. The CTS paper covers strategic, feasibility, economic, and cultural aspects of Phase 0 approaches, including topics such as the regulatory framework, analytical tools, and ethical considerations. Case studies and a review of the literature are provided in the Supplemental Information.
Adoption of Phase-0 approaches by industry has been limited, in part, by concerns about ‘false negatives’, costs, and delays to timelines often provided as reasons. While ‘false negatives’ are a valid concern, they apply to all phases of clinical development and especially the early, proof of concept studies that are typically underpowered. Importantly, Phase-0 may improve on an otherwise ‘false negative’ liability of pre-clinical candidate selection due to the non-human-specific sources of the data. Costs with Phase-0 are typically minimal relative to the value of the data but should be considered on a case-by-case basis. A comprehensive economic analysis of a range of developmental scenarios is upcoming by the authors. Concerns about delays to developmental timelines are largely unfounded as it has been demonstrated that Phase-0 studies can be conducted in parallel to preparations for Phase 1 and the transition from Phase 0 to Phase 1 can be accomplished using efficient adaptive designs.

The comment feature is locked by administrator.