Author: Sara Van Driest, MD, PhD on May 20, 2022 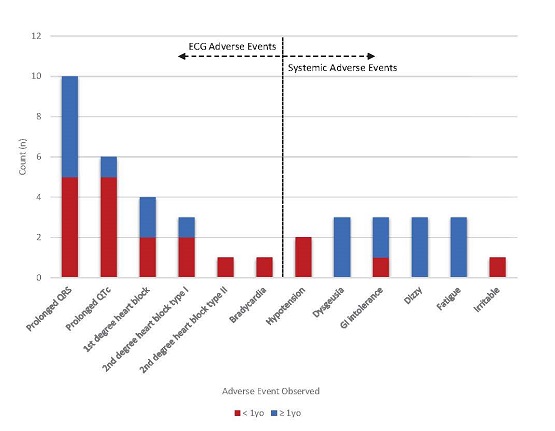
Using genetic test results to guide drug therapy (pharmacogenetics) is a cornerstone for the growing field of precision medicine. This approach has been implemented by some medical centers for an array of medications where the association between genetic variation and drug response has been well-established. However, many precision medicine implementations have a “one size fits all” approach. Specifically, the critically important variable of patient age is not assessed. For many drug-gene interactions, there are limited data from infants, children, and adolescents; given the vast biologic, physiologic, and metabolic changes between birth and early adulthood, pediatric-specific data are necessary to investigate the impact of age on drug response and pharmacogenetic associations.
A recent CTS paper by Sunthankar et al. reports the results of an investigation that set out to validate the association of CYP2D6 metabolizer status on response to the antiarrhythmic drug propafenone. Despite small sample size (n = 76), the association of slower CYP2D6 metabolism with increased risk for systemic side effects, previously seen in adults, was confirmed in this cohort of young individuals (age 0-27). Further, the investigators found that those older than 1 year of age were more likely to have adverse events with propafenone than those under 1 year old. These results support the use of pharmacogenetic data to predict risk of side effects with propafenone. More broadly this work sets an example for increased precision in pharmacogenomics by incorporating genotype and age into therapeutic decision making.

The comment feature is locked by administrator.