Author: Paulien Ravenstijn, PhD on August 23, 2022 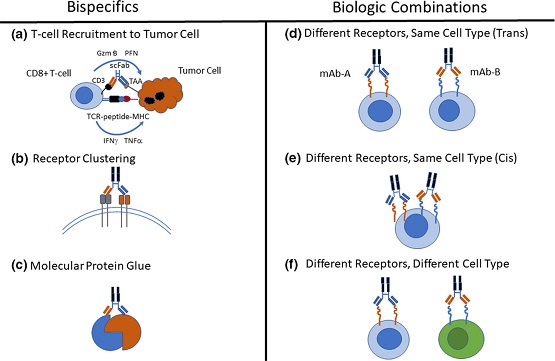
Targeting multiple mechanisms of action is a popular choice for biological drug development as it aspires to improve efficacy and safety, increase duration of response, and minimize resistance. The list of bispecific antibodies (BsAbs) with regulatory approval is growing. Next to building multiple targets in one molecule, combinations of biological drugs are also successfully being developed and approved as treatment options. But it does not stop there. Trispecific antibodies and BsAbs in combination with other biologics are also on the horizon and being considered as treatment options to maximize the clinical benefit.
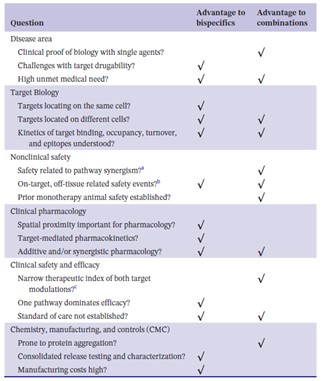
Table 2. Checklist of questions when deciding to develop biologic combinations or bispecific antibodies. (Zheng et al.)
“How to choose between these two approaches?” is a question that Zheng et al. asked themselves in a recently published mini-review, where they summarize examples of BsAbs and biologic combinations that have been approved by health authorities. They discuss the different drug development considerations when deciding between these two strategies, i.e., the advantages between the two strategies in terms of target biology, clinical pharmacology, non-clinical and clinical safety, efficacy, etc. The authors also address the question “When to consider a BsAb given the potential dosing challenges to optimize dosing … compared with a combination approach of two mAbs for which varying dose-ratios can be explored to achieve the optimal therapeutic benefit?”, which may have become a more important point for consideration in oncology given the recent introduction of the US Food and Drug Administration’s Project Optimus. The review also nicely compares the focus of clinical pharmacology in BsAbs development and combination drug development whereby the latter may have an advantage when there is previous knowledge on PK, PD, and dose-response from the individual monoclonal antibodies in the combination. Nevertheless, the authors conclude that BsAbs may still be in the running when there is a clear efficacy or safety advantage over the combination therapy, emphasizing the capabilities of BsAbs in cell-cell and protein-protein bridging or receptor clustering.

The comment feature is locked by administrator.