Author: Deanna L. Kroetz, PhD on September 23, 2022 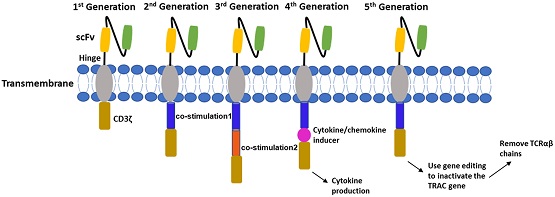
Engineered chimeric antigen receptor (CAR) T cells are the latest tool in the immunooncology arsenal, and there is much excitement around their clinical applications. In the last five years, six CAR-T cell therapies have been approved in the US for treatment of hematological malignancies. While the idea of using a patient’s own T cells to engineer an effective cancer treatment is exciting, it has required rethinking the typical pipeline for clinical pharmacology and toxicity assessments that are standard for small molecule and antibody therapies.
In a recent issue of Clinical and Translational Science, Johannes Kast, Vijay Upreti, and their colleagues present a detailed history of CAR-T therapies and discuss their clinical evaluation. With the explosion of cell therapy companies in recent years, the role of clinical pharmacology and translational scientists in developing safe and effective therapies cannot be understated. This is a must read for anyone faced with decisions around characterizing cellular kinetics, dose-response relationships, and toxicities. The review includes a detailed discussion of first-in-human dose selection and the application of modeling and simulation in the drug development process.
As cellular therapies are refined and barriers that currently prevent their effective use in solid tumors are overcome, the need for clinical pharmacologists and translational scientists to ensure that these therapies are safe and effective will only increase. Start your education now and become a part of this revolution.

The comment feature is locked by administrator.