Author: Sandhya Girish, PhD on October 04, 2022 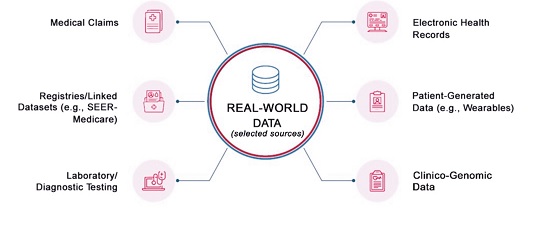
Pharmaceutical products in the current accelerated drug development landscape can benefit from tools beyond data generated from clinical trials. We have seen an abundance of real-world data (RWD) and real-world evidence, driven by the digitalization of healthcare systems and an increased awareness that has inspired a heightened interest in their potential use.
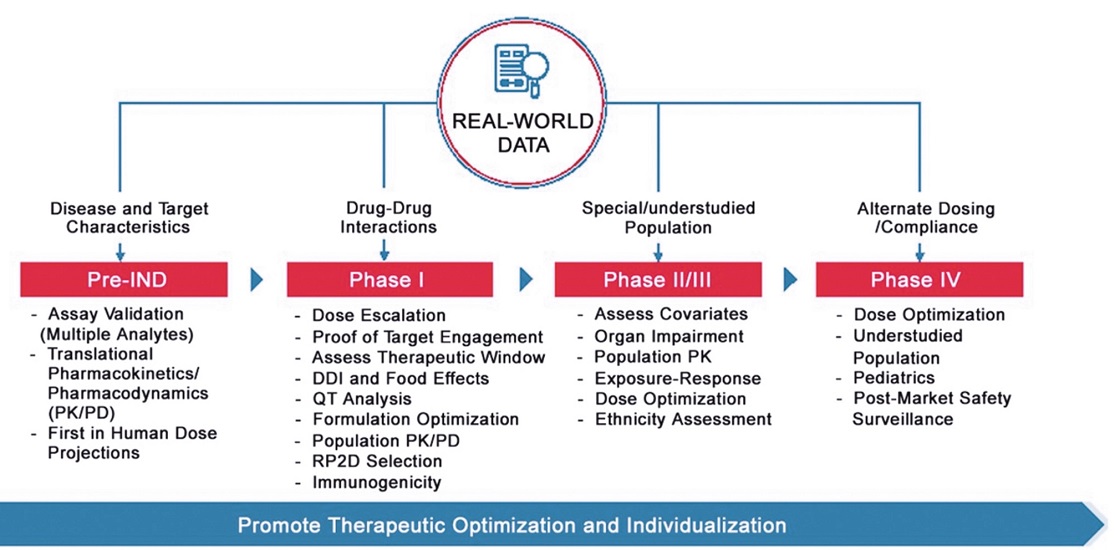
Clinical and Translational Science recently published an article by Molly Zhao, Shahed Iqbal, Ivelisse Valdes, Mark Dresser, and Sandhya Girish entitled “Integrating real-world data to accelerate and guide drug development: A clinical pharmacology perspective” that highlights promising examples in which RWD have been used to complement clinical pharmacology throughout various phases of drug development; case examples include dose/regimen extrapolation, dose adjustments for special populations (organ impairment, pediatrics, etc.), and pharmacokinetic/pharmacodynamic models to assess impact of various factors on treatment outcomes.
The authors also discuss limitations and promises of RWD to answer key scientific questions in drug development and articulate challenges posed by quality issues, data availability, and integration from various sources as well as the increased need for multidimensional-omics data that can better guide the development of personalized and precision medicine.

The comment feature is locked by administrator.