Author: Dhruvitkumar Sutaria, PhD and Rucha Sane, PhD on February 14, 2023 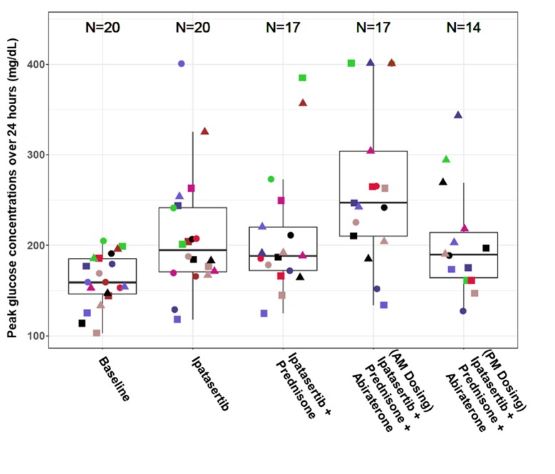
The FDA has recently approved several wearable technologies for monitoring at-home, including continuous glucose monitoring devices. Historically, clinical glucose monitoring has been carried out using either a finger prick or venipuncture, in which the sample is eventually evaluated by a specialized laboratory. The disadvantage of finger prick or venipuncture method is that it only measures glucose levels at a single moment in time. However, glucose changes are dynamic in nature, and can vary based on the meal intake or drug administration. Implementation of such continuous glucose monitoring devices within the clinical programs where hyperglycemia is a concern is still limited in application.
In the clinical study recently published within Clinical and Translational Science (CTS), Dhruvitkumar Sutaria, Rucha Sane, and colleagues employed the Dexcom G6 continuous glucose monitoring device to evaluate the real-time changes in glucose levels post-ipatasertib (AKT inhibitor) administration as well as ipatasertib in combination with prednisone (glucocorticoid) and abiraterone (androgen receptor inhibitor). The three drugs were added to the regimen sequentially to assess the reason for change in blood glucose and several glucose parameters such as peak glucose (shown in the figure above), average glucose, and percentage time in range were evaluated. Furthermore, since food intake can alter blood glucose, authors also evaluated the influence of morning versus evening dosing of ipatasertib in combination with prednisone and abiraterone.
Increased ipatasertib exposures in combination with abiraterone due to drug-drug interactions resulted in increased overall glucose profiles. The results from this study suggested that offering ipatasertib, an AKT inhibitor, as an evening dose after the evening meal may offer a better option for de-risking hyperglycemia. The study also provided preliminary information that a low dose of steroids does not lead to a marked increase in glucose levels when administered in combination with ipatasertib. Detailed study design and results are available in the full paper “Mitigating ipatasertib-induced glucose increase through dose and meal timing modifications.”
This article should be of great interest to cancer patients who are dealing with hyperglycemia as an on-target adverse reaction and their oncologists. This will also be of high interest to clinical pharmacologists, as this is the first clinical study wherein dynamic glucose changes were investigated using an innovative continuous glucose-monitoring wearable device to assess safety of ipatasertib. The results from this study suggested that offering ipatasertib as an evening dose in combination with prednisone and abiraterone followed by overnight fasting could offer a way to mitigate risk of hyperglycemia.

The comment feature is locked by administrator.