Author: Ann-Cathrine D. Dunvald, MD, PhD on August 21, 2023 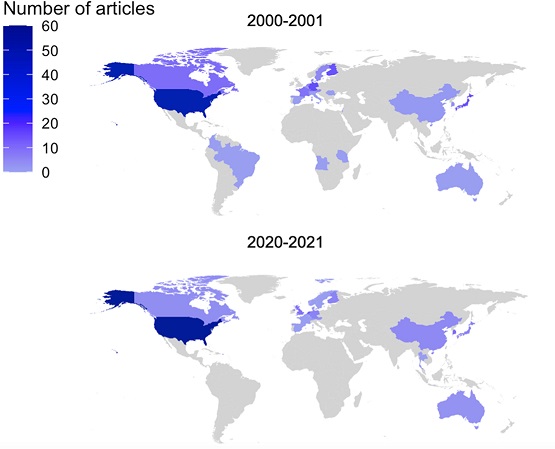
A few years ago, when our research team started a new sequence of clinical trials, we came to discuss whether or not we should include trial participants of both male and female sex. Historically, there have been concerns about enrolling females of childbearing potential in clinical trials, which has ultimately led to exclusion of females from early drug development studies. But, when clinical trials are conducted only with male trial participants, it is of concern that the information produced does not adequately shed light on the efficacy and safety of drugs in the general population. In our studies, we decided to include both female and male participants to account for potential sex-specific variation in, e.g., pharmacokinetics.
While the concerns regarding lack of female trial participants has been an issue for several years (and for example is addressed in this US Food and Drug Administration (FDA) guideline from 1993 ), the need to enroll trial participants from racial and ethnic minorities has gained increasing attention in the past years. It is a fact that some diseases are unequally distributed among races and ethnicities, and it is of the utmost importance that clinical trials reflect the population in which the medicinal product is intended to be used. Furthermore, a number of demographic characteristics may affect the clinical pharmacology, efficacy, or safety of a product. In 2022, the FDA issued a set of recommendations for sponsors to enhance clinical trial diversity, which outlines recommendations to assess the impact of racial and ethnic diversity throughout drug development.
In a recent review in Clinical and Translational Science (CTS), Julie Bøttern and colleagues aimed at mapping the current state of sex, ethnic, and racial diversity in clinical trials. They reviewed all papers of clinical trials published in CTS or Clinical Pharmacology & Therapeutics in 2000-2001 and 2020-2021, to assess the potential differences during the past 20 years. While the review shows that the proportion of female trial participants and participants from racial minorities increased in 2020-2021 compared to 2002-2001, males are still vastly over-represented while some races are under-represented. Since diversity is a translational and clinical research imperitive, awareness regarding the under-representation of women and minorities is essential in combatting the lack of diversity.

The comment feature is locked by administrator.