Author: Mohamed H. Shahin, BPharm, MS, PhD, MBI on January 11, 2024 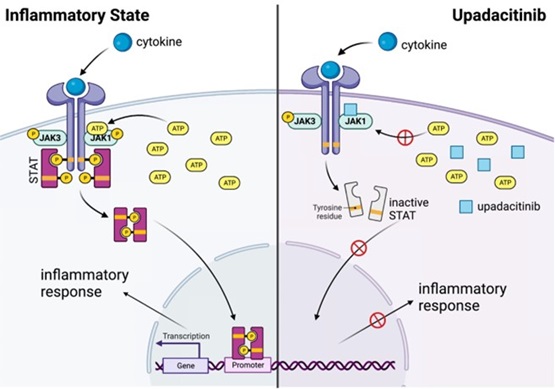
The world of biopharmaceuticals is ever evolving, with groundbreaking drugs constantly being developed to combat a myriad of diseases. Understanding how these drugs work, their pharmacokinetics, and pharmacodynamics is crucial not just for healthcare professionals but especially for anyone with an interest in clinical and translational science. That is why Clinical and Translational Science (CTS) is thrilled to announce our new series of mini-reviews, which aims to demystify these complex agents, from mechanism to development and regulatory journey to clinical impact.
Why This Mini-Review Series Matters
For practitioners, pharmacists, and researchers, these mini-reviews offer a concise yet comprehensive understanding of new therapeutics. For patients and the general public, they provide insights into how these drugs can manage or cure diseases, enhancing informed healthcare decisions. All the current CTS mini-reviews are available here.
Today’s Spotlight: Upadacitinib's Mechanism of Action
The figure above illustrates the mechanism of action of Upadacitinib, a selective Janus kinase (JAK) inhibitor used in the treatment of several chronic inflammatory diseases, including rheumatic, dermatologic, and gastrointestinal diseases. Such visuals are a cornerstone of our reviews, helping to translate complex pathways into understandable and memorable information.
Beyond the Image: In-Depth Analysis
In each mini-review, the authors delve into the crucial stages of drug approval, discuss the drug's pharmacokinetics/pharmacodynamics characteristics, and summarize key clinical trials. They also present clinical efficacy and safety, providing a well-rounded view of the drug's profile. If you would like to learn more about Updacitinib, check out this mechanism of action mini-review here.

The comment feature is locked by administrator.