Author: Vincent Aranzana-Climent, PhD, PharmD; Wisse van Os, MS; and Lena Friberg, PhD on July 02, 2024 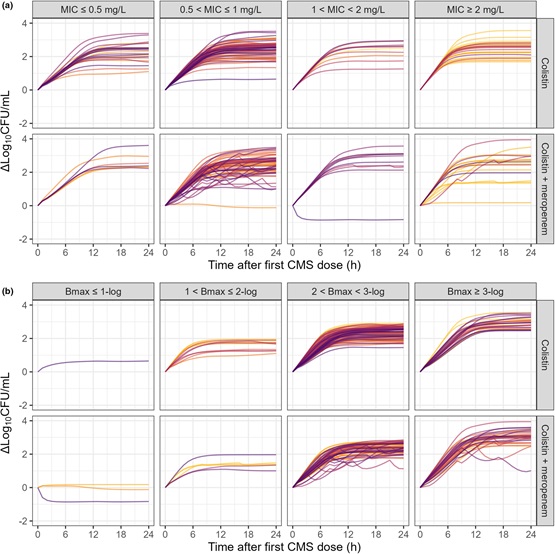
Given the accelerated antimicrobial resistance globally, which caused 1.27 million deaths in 2019, there is an urgent need for efficient methods that can speed up drug development and translate between preclinical and clinical settings. In antimicrobial pharmacokinetics-pharmacodynamics (PKPD), the clinical data obtained is usually limited to dichotomous measures of clinical and microbiological outcomes. These outcomes are relevant, but they provide no detailed quantitative information. Unfortunately, following the bacterial load during the treatment of patients is currently not feasible. On the contrary, preclinical experiments are more straightforward to perform; although, it is not always clear how well the measurements translate to clinical settings. Therefore, since we had access to a unique dataset where various preclinically PKPD experiments had been performed on the infecting bacteria in a clinical trial, we set out to examine potential associations between the predicted bacterial load and clinical outcomes.
Our study leverages results from the AIDA trial, wherein colistin was compared to colistin + meropenem against gram-negative pathogens. From the patients, we had data on individual colistin PK measurements, dosing information, demographical data, and microbiological and clinical outcomes. In addition to the clinical data, we had access to a variety of preclinical data on the bacterial strains isolated from each patient, namely the susceptibility to colistin and meropenem as well as the pharmacodynamic interaction between the drugs in vitro, and the in vivo bacterial fitness. All these data were integrated into a PKPD model to predict bacterial load over time in patients. We then explored whether these predictions were correlated with clinical and microbiological outcomes. We found that a 100-fold increase in predicted bacterial load over 24 hours was associated with a higher risk of clinical failure.
We believe that this analysis is a step towards more refined methods for model-assisted translation of antimicrobial effects, considering differences in patient demographics and disease severity. Applying the methodology to similar clinical study data will contribute further knowledge of value to facilitate early prioritization of promising antimicrobial treatments that are much in need.

The comment feature is locked by administrator.