Author: Marjoleen JMA Nijsen, PhD; Fan Wu, PhD; and Mary E. Spilker, PhD on February 22, 2018 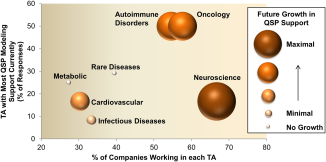
An IQ Consortium Survey
The cost of developing drugs is rising rapidly, and the number of new drug approvals per dollar spent showed a decline over the last decades. Approximately 90% of investigational drugs fail before being approved for use in patients, mostly attributed to either lack of efficacy or to drug-induced safety issues. The Critical Path Initiative led by the US Food and Drug Administration (2004) proposed the utilization of model-based approaches to improve drug development decision-making and, as a result, model-based drug development has evolved since the publication of the FDA report.
A survey conducted in 2014 by the preclinical Pharmacokinetics-Pharmacodynamics (PKPD) Discussion Group, assigned by the Drug Metabolism Leadership Group (DMLG) within the International Consortium for Innovation & Quality in Pharmaceutical Development (IQ), revealed that preclinical PKPD modeling is widely used in the pharmaceutical industry, enabling the selection and optimization of human doses and/or dose regimens, including prediction of human efficacious doses. While PKPD modeling approaches have proven invaluable, their utility in translating biological effects between species and their ability to rigorously assess the mechanism of action of novel drugs is limited. Since most current drug-discovery efforts are targeted toward complex diseases, understanding the pathophysiology at a systems level has been recognized as an important aid in target validation, biomarker selection, (pre)clinical study design, and patient stratification aimed at achieving higher success rates in clinical trials.
From the same PKPD survey, it can be concluded that the consistent use of more complex models, including systems pharmacology models, was less common compared to traditional PKPD models. Quantitative systems pharmacology (QSP) is an approach to describe the dynamic interactions between a therapy and a biological system in order to elucidate the behavior of the system as a whole, rather than just its individual constituents. Furthermore, it provides a quantitative or computational framework for formalizing knowledge about (patho)physiological and therapeutic mechanisms, as well as integrating data from different types of experiments and knowledge on biochemical, biological, physiological, pharmacological, and clinical systems.
To better understand the current landscape in preclinical QSP modeling and to stimulate the exchange of knowledge in QSP practices across the biopharmaceutical industry, a preclinical QSP Working Group within the IQ DMLG was formed in 2016, consisting of representatives from 17 pharmaceutical companies, ranging from small biotech to large pharma companies. One of the objectives of the preclinical QSP Working Group was to understand current challenges and opportunities for preclinical QSP modeling within R&D, as well as to evaluate the organizational structures of preclinical QSP modelers within the industry and their interface with other functional experts across R&D and regulatory agencies. Therefore, a new survey was conducted across 50 pharmaceutical companies in the first half of 2017, the results of which were recently published in PSP. Most of the companies that indicated they do not practice QSP modeling are small companies, suggesting that larger companies enable more opportunities to explore QSP tools and invest more resources to implement QSP approaches. Among the companies that use QSP modeling, QSP models are used in various ways (including mechanistic PBPK and PKPD), and for the majority of respondents, a clear definition of QSP is lacking.
In line with the wide variety of models defined as QSP, several software tools and languages are used, such as systems modeling tools, PBPK and PKPD software, as well as general engineering, computational, or statistical languages. Larger companies seem to be the trend setters in terms of preclinical QSP modeling support (starting around 2005), and QSP efforts really emerged after 2011 across companies. Not surprisingly, the success of QSP modeling is related to the number of years the company is exposed to QSP and the amount of full time employees assigned to QSP modeling support. A clearly defined problem with intended scope was identified as an essential factor of success and timely delivery of the QSP model outcome. Lack of interest from management was highlighted as reason for failure.
Overall, QSP modeling is recognized as being impactful and the majority of companies expect an increase in hiring and training of QSP modelers in the near future. In terms of the organizational structure, the survey results show that in most companies, preclinical QSP modelers report into DMPK and/or preclinical PKPD groups. In most cases, QSP modelers (either preclinical or clinical) have other job responsibilities, related to DMPK, Clinical Pharmacology, Pharmacometrics, or PKPD modeling, which is in agreement with the observed diversity in academic background across QSP modelers. Respondents indicated that the therapeutic focus is on oncology and immunology, and as such, most companies currently apply QSP models in these therapeutic areas. While a significant number of companies focus on neuroscience as a therapeutic area of interest, QSP modeling is currently not widely applied in neuroscience. As expected, a decent portion of companies do see future potential for QSP support in this therapeutic area.
Further, companies seem to start with QSP modeling before the selection of clinical candidates, mostly to interpret preclinical data sets, inform biomarker translation, and support clinical development of drug candidates, emphasizing the emerging direction of the preclinical QSP field. Given the mechanistic nature of QSP modeling, it is somewhat surprising that preclinical QSP isn’t routinely applied in the target identification/validation stage or for the assessment of safety issues.
In summary, the results of this survey show that preclinical QSP modeling is used across the majority of (mainly larger-sized) pharmaceutical companies and seems to be an emerging field with expected growth in the near future. The survey outcome indicates a clear need for a better definition or terminology around QSP, and one of the future objectives of the Working Group is to organize a QSP educational session and publish a follow-up manuscript to 1. review current applications of preclinical QSP modeling in pharmaceutical industry, 2. provide a general consensus in terminology around QSP, 3. showcase examples illustrating the impact of preclinical QSP modeling in drug development, and 4. discuss best practices and future opportunities (e.g. target identification, safety assessment, regulatory submissions).
This survey was developed with the support of the International Consortium for Innovation & Quality in Pharmaceutical Development (IQ). IQ is a not-for-profit organization of pharmaceutical and biotechnology companies with a mission of advancing science-based and scientifically-driven standards and regulations for pharmaceutical and biotechnology products worldwide. Consistent with this mission, it is the hope of the IQ DMLG QSP WG that the survey outcome will provide an understanding of the landscape of QSP across the Pharmaceutical Industry and its role in drug discovery and development, and will lead to further dialogues on the value and application of this approach to improve the probability of clinical success for future drugs.
Image by Nijsen, et al. CPT Pharmacometrics Syst. Pharmacol., doi: 10.1002/psp4.12282, is licensed under CC BY-NC-ND 4.0. ©2018 The authors.

The comment feature is locked by administrator.