Feb24 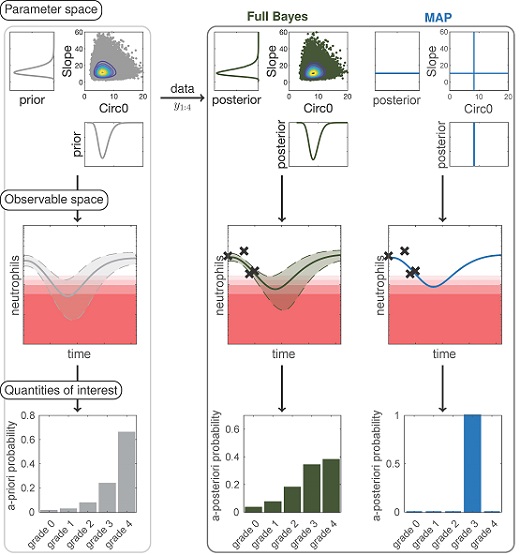 Huisinga Receives 2021 PSP Award
Huisinga Receives 2021 PSP Award
The editorial team of CPT: Pharmacometrics & Systems Pharmacology ( PSP ) is very pleased to present the 2021 PSP A ward to Wilhelm Huisinga, Professor of Mathematical Modelling & Systems Biology at the University of Potsdam/Germany for his article on " Bayesian data assimilation to sup...
Read More..
Dec03  PSP Presents the WCoP 2020 Abstracts
PSP Presents the WCoP 2020 Abstracts
CPT: Pharmacometrics & Systems Pharmacology ( PSP ) is honored to announce the publication of a special supplement: the World Conference on Pharmacometrics (WCoP) 2020 abstracts . We know we speak for many when we say how disappointed we were that we were not able to gather in Cape Town, Sou...
Read More..
Sep15  MIDD Guidance by Chinese NMPA
MIDD Guidance by Chinese NMPA
On August 3, 2020, China’s National Medical Products Administration (NMPA) announced Draft Technical Guidelines for Model-informed Drug Development (MIDD) . This document provides guidance on the rational use of MIDD to promote innovation and efficient drug development in China. This is a welc...
Read More..
Aug29  Advancing the Use of Mathematical and Computational Approaches in Drug Development
Advancing the Use of Mathematical and Computational Approaches in Drug Development
Many readers of PSP are members of the International Society of Pharmacometrics (ISoP) . Those who are not members are at least likely to be familiar with the society’s activities, such as the American Conference on Pharmacometrics (ACoP). Even readers who are active members of the society...
Read More..
Jul12 .png) Two NONMEM Tutorials in PSP!
Two NONMEM Tutorials in PSP!
NONMEM remains the most widely used software for population PK and PKD analyses, both within academia and in pharmaceutical industry. I believe the continuous development to include features requested by users, and various contributions to the pharmacometrics community, are part of the continued su...
Read More..
Jun25  ASCPT 2019 Annual Meeting Pre-conferences
ASCPT 2019 Annual Meeting Pre-conferences
The June 2019 issue of PSP is dedicated to articles contributed by the ASCPT 2019 Annual Meeting Pre-Conference speakers and organizers of “ PBPK (Physiologically-based Pharmacokinetic) Modeling for the Development and Approval of Locally Acting Drug Products ” and “ Advancing QSP (Quanti...
Read More..
May30 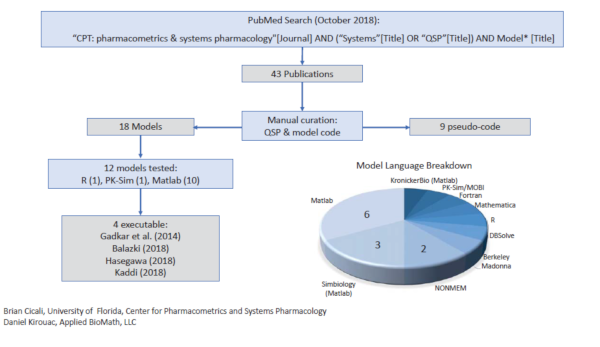 Establishing Reproducibility of Quantitative Systems Pharmacology Models
Establishing Reproducibility of Quantitative Systems Pharmacology Models
As the use of quantitative systems pharmacology (QSP) models is seeing widespread models – the publication by Kirouac et al., 2019 raises the issue of reproducibility . The authors conducted a survey of published models ( see Table 1, Kirouac et al., 2019 ) and found that after evaluating 12 m...
Read More..
Apr24 .png) Barriers to Unleashing the Full Potential of Model-Informed Drug Development
Barriers to Unleashing the Full Potential of Model-Informed Drug Development
A recent industry perspective by Jain, et al. identified barriers to unleashing the full potential of Model-informed Drug Development (MIDD). The barriers fall into two categories: the lack of acceptance by key stake holders, and the lack of consensus in standards among global regulators. In t...
Read More..
Mar12 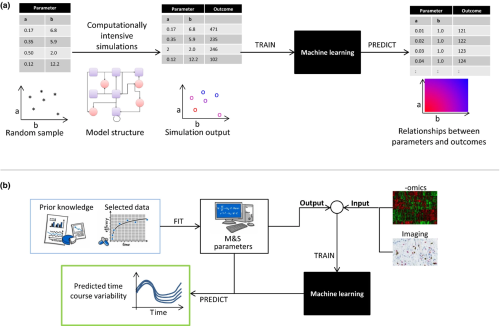 Can Machine Learning Improve Traditional Modeling Approaches in Clinical Pharmacology?
Can Machine Learning Improve Traditional Modeling Approaches in Clinical Pharmacology?
Machine learning (ML) and, specifically, deep learning (DL) algorithms are increasingly being used in the biological and health sciences to build predictive algorithms and to find associations between predictive features and outcomes. Examples of ML/DL applications are plentiful in areas as diverse...
Read More..
Feb25  Announcing CPT: Pharmacometrics & System Pharmacology 2.0
Announcing CPT: Pharmacometrics & System Pharmacology 2.0
After taking over the role of Editor-in-Chief of CPT: Pharmacometrics & Systems Pharmacology ( PSP ) in October 2018, one of my first tasks was to settle on a new editorial team. It is my distinct pleasure to present the team now:
Lena Friberg, PhD, of Uppsala University has agreed to ...
Read More..