Author: Ping Zhao, PhD on April 18, 2018 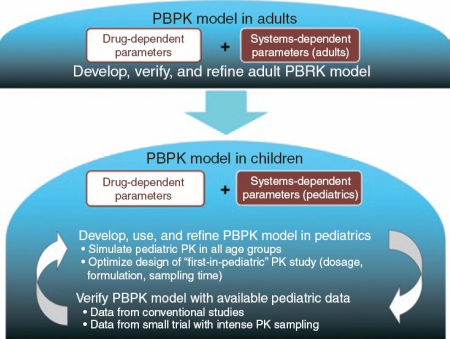
On April 11, 2018 the US FDA published a new Guidance for Industry “ICH E11(R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population,” introducing the role of modeling and simulations (M&S) in pediatric drug development. The addendum appears to expect that systems models consider “all available and relevant sources of existing knowledge” for pediatric assessment. Existing knowledge includes results in adults and other pediatric populations, nonclinical findings, data about related compounds, disease pathophysiology, as well as developmental physiology of a target pediatric population.
Fitting the bill of systems pharmacology models is physiologically-based pharmacokinetic model (PBPK). PSP has published Tutorials on PBPK in drug development and PBPK for pediatric assessment. The extra steps are essential before the further use of the PBPK model for pediatric applications (development, verification, and utility of the PBPK model in children) (Figure 1).
The FDA’s 2014 workshop concluded that confidence of PBPK to prospectively predict pediatric PK, especially in young children, needs improvement. In the wake of the FDA’s introduction of M&S in its new pediatric guidance, we should check the current status on the predictability of PBPK in pediatric assessment. An earlier FDA report (2012) summarized that PBPK can be used to plan first-in-pediatric PK studies, optimize pediatric study design, and inform ontogeny; whereas predictability based on analyses of three drugs appeared mediocre. Since then, ontogeny of certain drug disposition pathways has been evaluated through modeling of individual drugs (e.g., sirolimus and acetaminophen), and improved predictability has been demonstrated for renally cleared drugs as well as for drugs metabolized by P450s in children.
These learn-confirm efforts using existing and emerging pediatric data are expected to enhance PBPK models, which can play more impactful roles in pediatric drug development.
Disclaimer: The views do not reflect those of the Bill & Melinda Gates Foundation.
Image by Leong, et al. Clin. Pharmacol. Ther., doi: 10.1038/clpt.2012.19 © 2012 American Society for Clinical Pharmacology and Therapeutics

The comment feature is locked by administrator.