Author: [AUTHOR] Published on 2/13/2026 12:00:00 AM“From Maximum Tolerated” to “Optimal Biologically Active”
In the chemotherapy era, dose selection was simple: push the dose until toxicity appears, then step back slightly - the maximum tolerated dose (MTD) became the default. But this philosophy doesn’t fit modern oncology. Targeted agents and immunotherapies often reach plateau efficacy below the MTD, meaning that higher doses add side effects, not benefit.
Recent FDA guidance (FDA-2022-D-2827) now emphasizes dose optimization as an integral part of early clinical development, not an afterthought at registration.
A Mechanistic Framework for Dose Optimization
A helpful way to think about dose optimization is by grouping oncology therapeutics based on their mechanism of action (MOA) and the type of clinical activity they exhibit.
Each class brings unique translational and clinical challenges that shape how dose finding should be approached:
These distinctions remind us that dose optimization is not one-size-fits-all — it depends on how the drug works, what kind of biological feedback loops it engages, and whether efficacy can be measured in single-agent setting.
Clinical Development Milestones
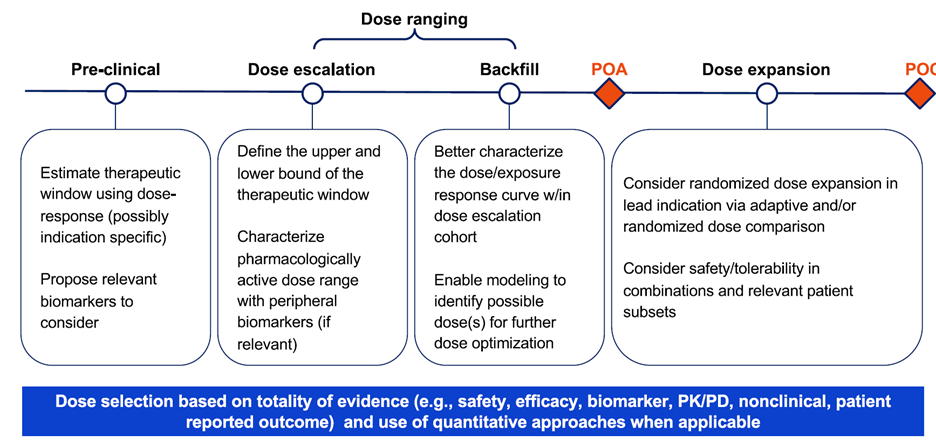
A structured approach to oncology dose finding involves two key checkpoints:
Proof of Activity (POA) – Demonstrating measurable antitumor activity (e.g., tumor shrinkage, ctDNA clearance). This is a gatekeeper for expanding development beyond dose escalation.
Proof of Concept (POC) – Showing efficacy relative to standard of care, enabling registrational study design.
In practice, dose selection should be based on the totality of evidence, safety, efficacy, biomarkers, PK/PD, patient-reported outcomes (PROs), and translational data, integrated through quantitative modeling whenever possible.
Model-informed Drug Development (MIDD) as the Backbone
Modern dose optimization is increasingly model-informed.
Depending on the molecule, developers may rely on:
- Population PK/PD and exposure–response analyses
- Physiologically based PK models
- Quantitative systems pharmacology models
These tools help integrate diverse datasets and enable rational, data-driven dose selection, aligned with FDA’s Project Optimus principles.
Practical Lessons for Developers
- Start early: Dose optimization should begin before phase 2, guided by translational modeling.
- Use POA as a gate: Expand only once biological or clinical activity is demonstrated.
- Incorporate PROs: Understanding patient tolerability improves long-term adherence and outcomes.
- Randomize doses when feasible: This helps mitigate bias and quantify benefit–risk trade-offs. Tailor by mechanism: What works for a cytotoxic or mAb won’t fit a T-cell engager or LAG-3 inhibitor.
Ref: Zhu J, Schroeder A, Frank S, Boetsch C, Jamois C, Kassir N, et al. 2025. Oncology Dose Optimization: Tailored Approaches to Different Molecular Classes. Clinical Pharmacology & Therapeutics. 118(1):74–79. doi:10.1002/cpt.3658
