Author: Naoto Uemura, MD, PhD on August 29, 2017 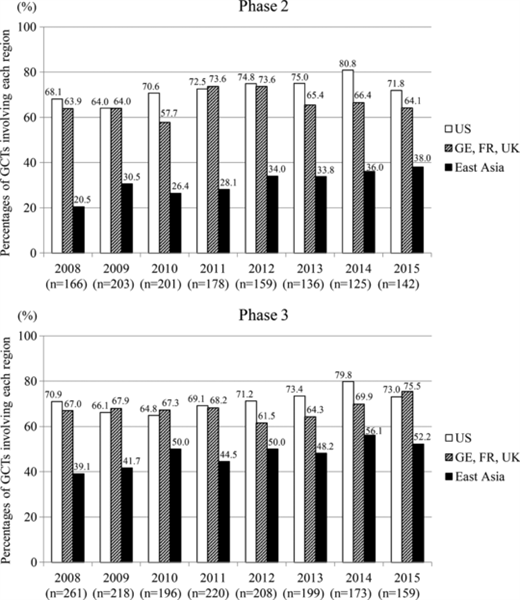
Today in 2017, multiple registration trials from phase I through phase III are being conducted at my institution, which is an academic research organization located in southern Japan. As late phase studies, especially phase III trials, tend to become larger, a number of such confirmatory studies are conducted as so-called "global clinical trials" (GCTs). It is very surprising to see few researchers apparently remember that global studies were almost impossible to execute in many therapeutic areas in Japan until 2007, after a series of meetings and discussions with experts to promote such GCTs resulted in the regulatory agency publication, Basic Principles on Global Clinical Trials.
If data from a pivotal study are not interpretable for an ethnic population in one particular geographic area, the investigational drug tested in that study may not be effectively marketable. When a local study is additionally required, the time between the local trial and the global trial may become the source of “drug lag.” Thus, GCTs need to be planned and executed very efficiently based on a good development strategy to allow all global patients access to good drugs without a delay.
Miyazaki et al. (2017), recently published in Clinical and Translational Science, acknowledged the establishment of global strategies in Japan and reported that contributions from East Asia to GCTs have gradually increased, in particular by Japanese pharmaceutical enterprises. The authors suggested the innovative concept of “pooled regions” proposed in the draft ICH E17 guideline may be applied to GCTs. The ICH E17 guideline is currently under development and highlights the importance of considering ethnic factors and confirming data consistency across different populations. A truly global harmonization should avoid duplication of similar trials and provide improved evidence for pooled population based on data efficiently collected across various regions/countries, not only from late phase but also from early phase studies.
Image by Miyazaki, et al. Clin. Trans. Sci., doi: 10.1111/cts.12485, is licensed under CC BY-NC 4.0. ©2017 The authors.

The comment feature is locked by administrator.