Author: Sarah M. Robertson, PharmD on September 26, 2018 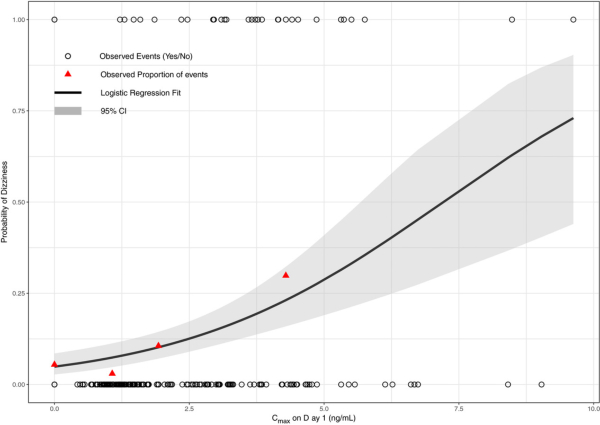
As our modeling colleagues in drug development remind us, exposure-response (ER) analyses are a critical component to proper dose selection, with the transition from phase II to phase III as a particularly critical juncture. Beyond maximizing efficacy, ER can be incredibly valuable for the interpretation of safety findings in clinical trials and to inform the risk assessment for a selected dose. In my experience, however, well-described ER relationships for adverse events are not common. Although we always look at exposure data in an effort to understand safety or tolerability findings, it’s not often that a clear ER relationship emerges.
The recently published paper from Cosson et al. in Clinical and Translational Science, Population Pharmacokinetic and Exposure-dizziness Modeling for a Metabotropic Glutamate Receptor Subtype 5 Negative Allosteric Modulator in Major Depressive Disorder Patients, stands out as a great example of an ER analysis for dizziness, with data informing both formulation development and dose selection for phase III. In this case, mitigation steps resulting from the ER analysis included both a modified release formulation to blunt Cmax and dose titration.
And for my fellow drug metabolism enthusiasts, the population PK modeling results described in the manuscript unveiled some interesting covariate effects for this drug, which is metabolized by CYP1A2. Happy reading!
Image by Cosson, et al. Clin. Trans. Sci., doi.org/10.1111/cts.12566, is licensed under CC BY-NC-ND 4.0. ©2018 The authors.

The comment feature is locked by administrator.