Author: Mark J. Dresser, PhD on May 20, 2019 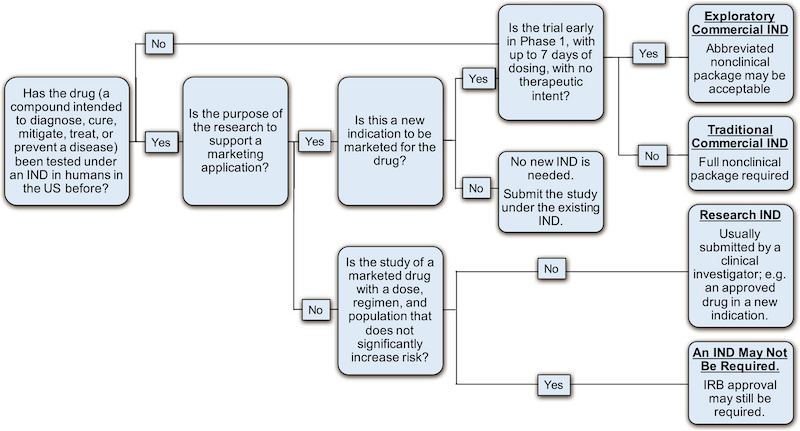
As part of CTS’s continued commitment to providing practical, open access tutorials on tools, methodologies, and approaches in translational medicine, the CTS editorial team is delighted to highlight the first in a series of Regulatory Affairs tutorials. The tutorial, Regulatory Affairs 101: Introduction to Investigational New Drug Applications and Clinical Trial Applications provides an excellent introduction to this important and complex topic. We anticipate it will be of high interest to anyone wanting to learn about and understand the information required by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) to initiate and conduct medical experiments in human subjects.
Many critical topics are covered, including the data required to support first-in-human administration of a new experimental drug such as the data and documentation describing how the drug is made (chemistry, manufacturing, and controls (CMC)), nonclinical and in vitro data characterizing the pharmacology, pharmacokinetics, and toxicology, and the proposed plans for evaluating the experimental drug for the first time in humans in the form of a clinical study protocol. This tutorial also addresses the key elements of the first-in-human (FIH) protocol such as the study population, dose selection, and the safety monitoring plan.
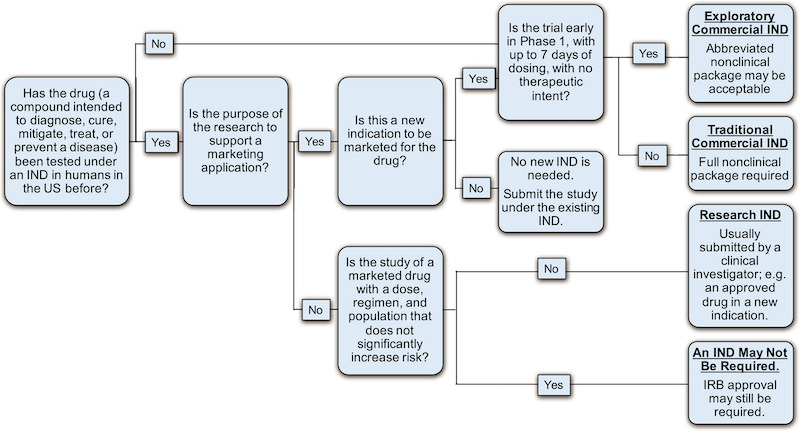
One of the major decision points in the process of preparing for first-in-human (FIH) testing of a new experimental drug, is whether to file an IND or a CTA. Many factors are taken into account for this decision. In this tutorial, the authors summarize the key similarities and differences of the two types of submissions, the health authority review and clearance (IND) or approval (CTA) process as well as the critical maintenance activities and responsibilities for the Sponsors once an IND or CTA is in effect.
We hope that this tutorial will serve as a helpful starting point for researchers new to the drug development process as well as those who have been working in the field but who have not yet had the chance to work on both INDs and CTAs. We also anticipate that this will serve as a useful refresher or point of reference for experienced researchers.
The CTS editorial team would like to thank the authors, members of the Bay Area Regulatory Network, for their fantastic contribution, and we are excited to announce that we have additional Regulatory Affairs tutorials in preparation for the second half of 2019 covering expedited pathways and CMC.
Image by Chidden, et al. Clin. Trans. Sci., doi: 10.1111/cts.12635 , is licensed under CC BY 4.0. ©2018 The authors

The comment feature is locked by administrator.