Author: Deanna L. Kroetz, PhD on June 14, 2019 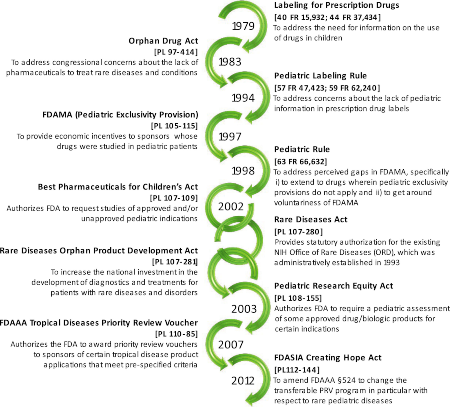
There is a saying in experimental pharmacology that preclinical species are not little humans. In clinical pharmacology, the same sentiment is true for pediatric populations. You do not need to be a parent or a pediatrician to realize that children are not just mini versions of adults. Yet a common mistake in pediatric clinical trial design is to just dose adjust for body weight and follow an adult protocol. With the adoption of several important federal regulations mandating appropriate studies of drugs in pediatric patients, these special populations are finally receiving the attention they need for optimal treatment of both common and rare medical conditions in children.
In the most recent issue of Clinical and Translational Science, Shakhnovich and colleagues provide a much-needed tutorial for conducting clinical pharmacology studies in pediatric populations. The authors are seasoned pediatric clinical pharmacologists and share their expertise in a thoughtful and detailed tutorial. Readers will be educated on key issues that distinguish adult and pediatric studies, including selection of dosing schemes and appropriate formulation, the influence of developmental regulation of drug metabolism and transport on pharmacokinetic and pharmacodynamic relationships, limitations of biological sample collection in children, selection of and access to an appropriate study population, the benefits of maturity markers vs age for patient stratification, and consideration of natural fears and concerns of both children and parents. The tutorial should be required reading prior to the development of a pediatric clinical pharmacology study by industry and academic scientists. Evidence-based treatment of children is critical for improving the lives of these special populations, and well-designed studies are needed to reach that goal.
The editorial leadership of Clinical and Translational Science, is devoted to the development of open access tutorials to contribute to the open education of our readers on best practices within the field. This important tutorial on conducting studies in pediatric populations is the first in a series of tutorials on clinical studies in special populations. We encourage suggestions from our readers on other important topics that are appropriate for our tutorial series.
Image by Shakhnovich et al. Clin. Trans. Sci., doi: 10.1111/cts.12615, is licensed under CC BY-NC 4.0. ©2019 The authors

The comment feature is locked by administrator.