Author: Sarah M. Robertson, PharmD on August 09, 2019 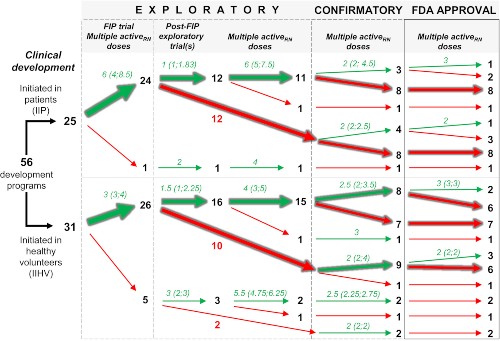
A recent publication in CTS by Lyauk et al. presents results from an evaluation of dose selection in new drug applications (NDAs) approved by the US Food and Drug Administration (FDA) between 2015 and 2017, in an effort to characterize the extent to which sponsors follow the “learn and confirm” paradigm of dose selection. The authors considered whether multiple doses were evaluated during the “learn” phase of development (i.e. Phase 2), the range of doses evaluated in Phase 2, the number of doses included in Phase 3, and whether results of dose- or exposure-response were referenced in the FDA review. Despite years of publications, panel discussions, and expert consensus on the importance of robust dose-finding and the value of model-based drug development, a little over half of dose-ranging trials included just two or three active doses over ≤5-fold range, suggesting that most trials are not designed or powered for model-based analysis. Further, a third of the FDA reviews did not report any exposure-response evaluation, and model-based dose/exposure-response were alluded to in only 2 of 60 approved indications. The authors theorize that drug developers are more results oriented (confirming) than performance (learning) oriented, leading one to wonder what we might find were this exercise to be carried out for unapproved NDAs. If we’re learning anything about dose selection, it might be the hard way.
Image by Lyauk et al. Clin. Trans. Sci., https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.12641, is licensed under CC BY-NC-ND 4.0. ©2019 The Authors.

The comment feature is locked by administrator.