Author: Charles Leonard, PharmD, MSCE, and Cheng Chen, PhD on May 23, 2023 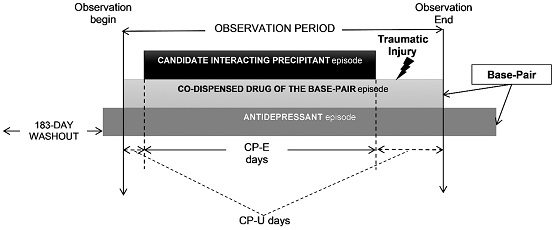
MOTIVATION
There is a high prevalence of mental illness and substantial utilization of psychoactive prescription drugs (including antidepressants) among Americans. Given the bidirectional relationship between mental illness and other chronic medical conditions, it is very common for persons with a mental illness to simultaneously use multiple prescription drugs. This "polypharmacy" increases their likelihood of experiencing harmful drug interactions—largely preventable events. Unfortunately, nearly all evidence on drug interactions (including with psychoactive drugs, like antidepressants) comes from case reports and/or pharmacokinetic (PK) studies examining drug concentration changes rather than health outcomes. A recent systematic review of purported psychoactive drug interactions rated as major or contraindicated across drug interaction compendia—a scenario where the best evidence should be found—determined that 31% of “interactions” were supported by no clinical outcomes data, and only 12% were supported by a clinical outcome study of >100 subjects. Thus, drug interaction compendia and computerized clinical decision support tools surely fail to warn against unrecognized harmful psychoactive drug interactions, and falsely warn against safe combinations that prevent appropriate use and contribute to alert fatigue. Can real-world data (RWD) help identify harmful drug interactions with antidepressants?
FINDINGS
Our Clinical and Translational Science (CTS) paper harnessed RWD to identify antidepressant drug-drug-drug interaction (3DI) signals of potential clinical importance. Our intent was to inform and prioritize follow-up etiologic studies that will confirm or refute the population health effects of antidepressant drug interactions that increase one’s risk of traumatic injury. We focused on 3DIs, i.e., an antidepressant taken simultaneously with two other drugs, since population-based data on drug interaction triads are particularly scarce. We selected injury as an outcome because previous research demonstrates that antidepressant use (compared to non-use) can elevate rates of fall-related injury, hip fracture, and motor vehicle crash; therefore, interactions among antidepressants and other medications may further amplify these inherent risks. For each antidepressant triad—consisting of an antidepressant + co-dispensed drug (base-pair) and a candidate interacting medication (precipitant)—we compared traumatic injury rates when exposed to the antidepressant triad versus when exposed to the base-pair alone, adjusting for confounding variables. We ultimately deemed antidepressant triads with increased injury rates as 3DI signals. Essentially, our approach aimed to answer the following question for each antidepressant base-pair: Which additional drug, when combined, is associated with an increased risk of injury?
Using healthcare data routinely collected for reimbursement purposes, we screened 120,714 antidepressant triads. Among these, 334 triads were associated with elevated injury rates and thus considered 3DI signals. Remarkably, nearly half of signals had confounder-adjusted rate ratios ≥ 2, suggesting at least a doubling of harm. Many signals had plausible PK and/or pharmacodynamic (PD) mechanisms, thus bolstering the validity of our findings. For instance, we observed the addition of divalproex to the use of escitalopram + memantine was associated with a 2.4-fold increased injury rate. This finding may be explained by a PD interaction among these three agents. Additionally, we uncovered 3DI signals that lacked an apparent biological mechanism. Notably, adding aripiprazole to the use of escitalopram + simvastatin was associated with a remarkable 6.6-fold increase in the injury rate, while the addition of clonazepam to escitalopram + olmesartan was associated with a 4.4-fold increase in the injury rate. These strong signals may warrant prioritization and further evaluation in future etiologic studies.
Our work demonstrated an approach to utilize RWD to generate hypotheses regarding clinically meaningful drug interactions. The 334 signals we identified represent antidepressant 3DIs of potential clinical concern and should inform future etiologic studies to test these hypotheses. We hope such work will improve the safety of pharmacotherapy provided to persons with an indication for an antidepressant.
CTS is currently inviting submissions for a special collection on real-world evidence (RWE)/RWD that is scheduled for initial, rolling publication in 2023. This RWE/RWD special collection is built on the foundation of the ASCPT March 21, 2023 preconference, Advancing the Utilization of RWD and RWE in Clinical Pharmacology and Translational Research, co-sponsored by the IQ Consortium. The preconference and the special collection aim to advance understanding and application of RWD/RWE. Selected content from the preconference will be highlighted in the CTS special collection; in addition, new original RWE/RWD articles are welcomed.

The comment feature is locked by administrator.