Author: Sebastiaan JW van Kraaij, MD and Matthijs Moerland, PhD on October 09, 2023 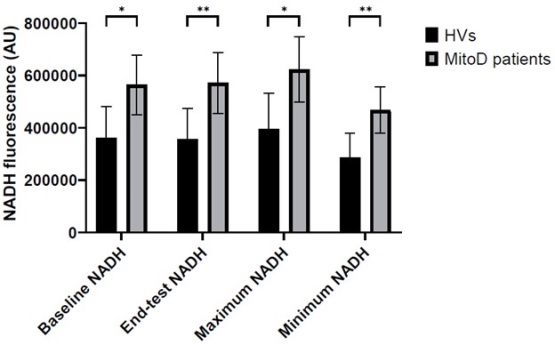
Developing efficacious treatments for rare disorders is a challenging endeavor, with an estimated 6% of drugs in development for orphan indications reaching approval, a figure about 75% lower than the pharmaceutical industry average.1 A good example is the field of mitochondrial disorders, for no unequivocally clinically efficacious treatment exists to date, despite numerous recently completed or currently ongoing clinical trials with drug candidates.2
Employing fit-for-purpose biomarkers in the earliest phases of drug development can advance rational clinical development of drug candidates. Identifying the right biomarker(s) for this task is crucial, especially in mitochondrial disorders—diseases characterized by substantial heterogeneity, both between and within patients (e.g., over time or between tissues) and an often incompletely understood pathophysiology. A holistic approach, which includes biomarkers at different physiological levels to capture the full range of mitochondrial dysfunction, i.e., cellular functional assays, circulating biomarkers and tissue-level assessments, may therefore be advisable.
A recent study by Van Kraaij et al. published in Clinical and Translational Science investigated the value of a panel of biomarkers in these categories, including a novel imaging technique that measures tissue-level mitochondrial dysfunction by assessing NADH fluorescence in the skin. Whereas this biomarker and several circulating markers of inflammation differentiated between patients and healthy controls, this was not the case for more proximal markers such as mitochondrial reactive oxygen species production or membrane potential. This finding contrasts with other studies in the field, where differences between patients and healthy volunteers in ex vivo mitochondrial functional assays were found. The authors hypothesize that the lack of differentiation in assays of mitochondrial function may be due to non-detectable differences in function on a cellular level coalescing into detectable changes on a tissue level, or that a new equilibrium is achieved in cells of patients with mitochondrial disease, necessitating a physiological challenge to reveal the underlying dysfunction. The study indicates that biomarkers assessing function on a tissue level, specifically imaging, may provide additional information not revealed by proximal biomarkers, and further research is needed to identify and validate distal biomarkers for use in early phase drug development, especially in complex and rare diseases such as mitochondrial disorders.
- Wong, C.H., Siah, K.W., & Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273-286 (2018). doi:10.1093/biostatistics/kxx069
- Almannai, M., El-Hattab, A.W., Ali, M., Soler-Alfonso, C., & Scaglia, F. Clinical trials in mitochondrial disorders, an update. Mol. Genet. Metab. 131, 1-13 (2020). doi:10.1016/j.ymgme.2020.10.002

The comment feature is locked by administrator.