Author: Aman P. Singh, PhD on February 06, 2025 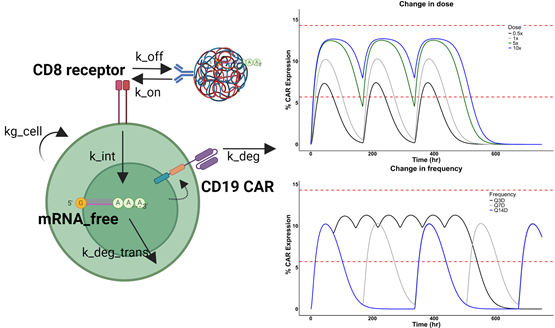
Figure 1. Left panel: Model schematic for the cell-level PK-PD model to characterize the transient CAR expression on T cells after NP-encapsulated mRNA transfection. Upon dosing mRNA in NP to the compartment with T cells, targeted binding of NP to T cells driven by the binding kinetics (kon, koff) results in internalization of the NP-receptor complex (kint) and subsequent intracellular release of mRNA (mRNA_free). The free, intracellular mRNA concentration drives the formation of CD19 CAR protein on the surface which subsequently degrades (kdeg_trans).
Right panel: Investigation of the effect of change in dose level and dosing frequency. The original dosing regimen is simulated in grey.
In vivo programming of endogenous T-cells to induce transient CAR-expression (in vivo CAR-Ts) could be a potential alternative to exogenously administered autologous and allogeneic cell therapies, which inherently possess several limitations such as logistical/financial constraints and graft vs. host diseases, respectively. Here we present a case-study of CAR-mRNA loaded targeted nanoparticle-based therapeutics to induce transient CAR-expression on endogenous T-cells. This novel drug delivery approach has mostly been evaluated in preclinical studies with only a handful of early clinical trials enrolling patients.
A translational pharmacokinetics-pharmacodynamics (PKPD) model was developed to optimize the dose and dosing regimen of these targeted nanoparticles to induce a sustained CAR-expression. The model accounts for physiological parameters and intracellular processing to characterize the transient CAR-expression on T-cells leveraging both preclinical in vitro and in vivo datasets. Moreover, global sensitivity analyses reaffirmed the importance of engineering stable mRNA constructs that exhibit prolonged intracellular PK profiles to have longer expression of proteins. Our simulations suggest that optimizing the dose and dosing regimen could lead to a sustained CAR-expression on endogenous T-cells despite the transient nature of mRNA expression.
Overall, our study highlights the potential of cell-level mechanistic PKPD modeling and a simulation approach in streamlining novel modalities such as CAR-mRNA loaded nanoparticles from preclinical studies to clinical applications in guiding first in human dose projections as these models could be integrated into whole-body physiologically base pharmacokinetcs frameworks.

The comment feature is locked by administrator.