Author: Arjun P. Athreya, PhD on June 20, 2025 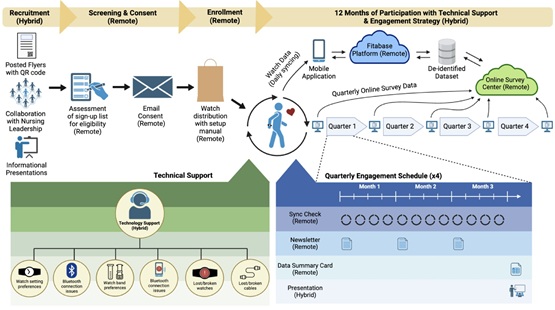
Digital Health Technologies (DHTs: comprising mobile applications, wearables, smart internet-connected devices) have the potential to inform patients’ functioning through remotely collected measurements and transform clinical trials with decentralized operations. Despite the strong potential, the efficacy of interventions with DHTs is met with low adherence (e.g., completing surveys, wearing devices). This report discusses novel participant-centered engagement strategies, in line with the US Food and Drug Administration’s guidelines on improving effective use of DHTs in clinical studies. Of interest, this study reports adherence to the use of smartwatches in a year-long wellbeing study, and participant perspectives on how DHTs improved or hindered their physical wellness/awareness.

The comment feature is locked by administrator.