Author: Florian Schmitzberger, MD on April 30, 2025 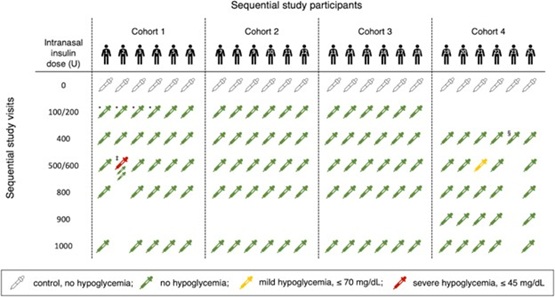
Intranasal insulin may have a use as a neuroprotective treatment for a multitude of diseases ranging from cardiac arrest to traumatic brain injury to Alzheimer’s disease. Animal data suggests that high doses of insulin may be required for neuroprotective effects. Our phase I study, involving healthy human participants, aimed to identify the maximum tolerated dose of intranasal insulin, exploring doses up to 1,000 units! Regular insulin was administered intranasally to 24 participants, and key physiological responses, such as serum glucose, insulin, and C-peptide levels were monitored. At all doses, including the high-dose, intranasal insulin was generally well tolerated, with minimal systemic absorption and predominantly stable glucose levels. The study identified two instances of hypoglycemia in 116 study drug administrations, suggesting that while most participants experience negligible changes, some may have a component of systemic absorption. Both episodes were easily managed.
Why is this research significant? Current neuroprotective treatments struggle to translate preclinical success to real-world clinical application. Among these challenges are logistical limitations of early intervention. Intranasal insulin's direct nose-to-brain delivery mechanism offers a potential swift and effective solution and positions it as a candidate for immediate post injury intervention by first responders.
Although further studies are necessary to refine dosage and application methods, this study lays the groundwork to inform future translational studies. What if intranasal insulin could augment treatment to reduce neurological outcomes secondary to insult?

The comment feature is locked by administrator.