Author: Yoonjin Kim, MD on August 12, 2025 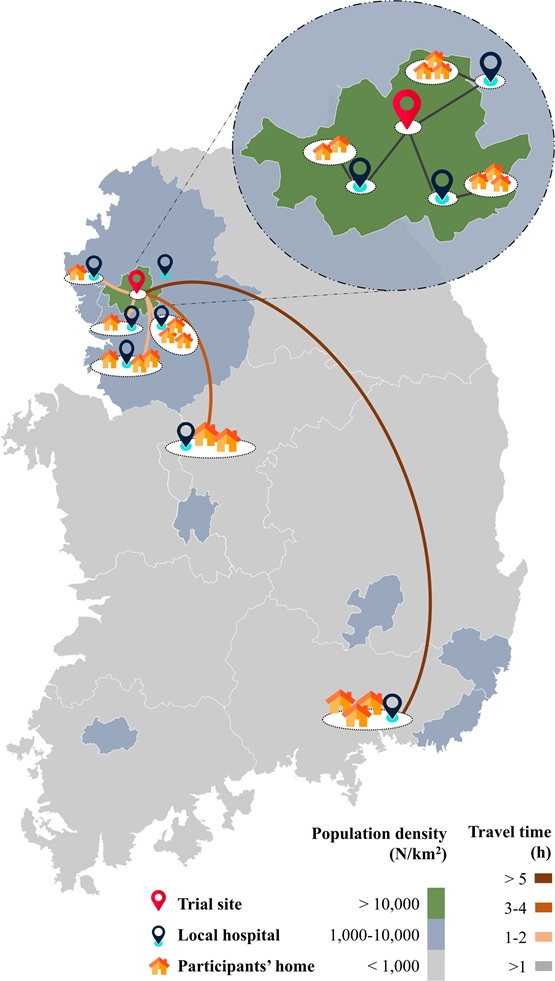
Data integrity remains a major challenge in decentralized clinical trials. Especially during eligibility assessment, ensuring comprehensive participant characterization is paramount in remote settings. To address this, our team conducted a randomized, open-label, single-center, fully remote trial in 20 healthy Korean adults, testing the feasibility of using personal health records (PHRs) for screening. Although most participants reported no significant medical history or prior medication use during initial interviews, review of their PHRs—including two-year health check-ups and three-month prescription records—revealed additional information in approximately half of the participants. Specifically, medical histories were identified in 9 participants (45.0%, 17 cases), and prior medication use in 10 participants (50.0%, 68 cases), underscoring the value of PHRs in strengthening data accuracy at the eligibility stage.
The study also tested other remote methods: e-consent, self-recorded diaries, smartwatch-based drug monitoring, and digital adverse event reporting. Most participants were satisfied, though some had trouble accessing PHRs or using the smartwatch. Korea’s infrastructure—efficient parcel delivery and accessible local hospitals—also played a key role in the trial’s success, suggesting that local infrastructure is crucial in decentralized research.

The comment feature is locked by administrator.