Author: Brittany Gonzales, Jose Rivera, PharmD, D. Max Smith, PharmD, BCPS on July 30, 2025 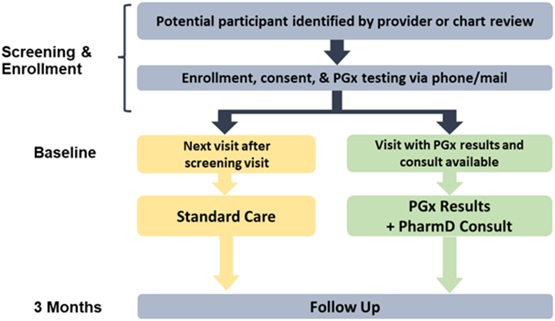
Chronic pain is a widespread issue that presents significant treatment challenges. There are a variety of pharmacotherapy options, with opioids among them. The two most widely prescribed opioids are hydrocodone and tramadol; however, their effectiveness is associated with CYP2D6 function, given the role of CYP2D6 in their metabolism to active metabolites. Clinical Pharmacogenetic Implementation Consortium (CPIC) guidelines provide recommendations for opioids and CYP2D6,1 but there have been limited randomized trials investigating prospective application of CYP2D6 to guide pain management. The PGx Applied to Chronic Pain Treatment in Primary Care Trial (PGx-ACT) examined this approach by integrating genetic testing into standard clinical processes.2
To date, PGx-ACT is the largest randomized trial investigating the effectiveness of PGx-guided pain management in chronic pain. The PGx-ACT trial was built upon findings from a prospective non-randomized pragmatic clinical trial by Smith et al. that found improved pain intensity among CYP2D6 intermediate or poor metabolizers (IM/PMs) in PGx-guided care versus standard care.3 Both trials had PGx-trained pharmacists provide consultation notes and education to aid providers in interpreting and applying PGx results. PGx-ACT made several changes from the prior trial, most importantly: A) randomization, B) CYP2D6 activity scoring in accordance with more recent guidelines1, C) inclusion of hydrocodone in the primary analysis.
PGx-ACT found similar pain symptoms between the CYP2D6 IM/PMs assigned to the PGx vs. standard care arms (p = 0.74). We suspect a primary cause for this was a lack of provider adoption of results. Prescribing decisions were at providers’ discretion, and when examining how often patients received care that aligned with their PGx results, there was no difference between groups.
PGx-ACT generated valuable insights, especially surrounding real-world utility regarding operations, provider engagement, and clinical decision support alerts. Operationally, mailing kits to patient homes resulted in many kits not being returned. Of the 315 patients enrolled, 42 (13%) were not randomized due to patients who did not return the PGx kits mailed to their homes. Regarding provider engagement, provider application of results may have been limited by the study protocol dictating to order the PGx test. For lab tests, providers are often trained, “if you order it, you use it." In PGx-ACT, they did not order it (essentially the protocol did) and did not use it. Additionally, applying PGx results takes practice. Providers were consulted and provided education prior to the study but had patients so infrequently (averaging 3 each over two years) that pharmacogenomics utilization was extremely limited per provider. Lastly, this trial occurred during the COVID-19 pandemic, which could have affected patient and provider engagement. The use of post-test clinical decision support (CDS) was infrequent (n = 5 participants), but invariably overridden, perhaps due to ineffective alert design or patient/provider unwillingness to change medication.
While this study illustrated that current PGx implementation into a real-world setting did not generate the desired results, it generated hypotheses on where to improve current strategies. There was a difference in a planned secondary analysis of poor and intermediate metabolizers who had >1 analgesic medication change: PGx-guided care was associated with a minor reduction in median pain intensity vs. standard of care, standardized difference. This trend carried through for improved physical function, pain interference, and greater reduction in morphine milliequivalents prescribed. Although preliminary, the clinical improvements are particularly promising as they occurred in the context of reduced opioid prescribing. Taken together, these results suggest that before any clinical benefits may come to fruition, we must first identify how to effectively incorporate PGx into care. This trial highlights challenges regarding the real-world uptake of PGx-guided care while also providing some insight of potential patient benefits.
References
1. Crews, K.R. et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6, OPRM1, and COMT genotype and select opioid therapy. Clin. Pharmacol. Ther., 110, 888-896 (2021).
2. Smith, D.M. et al. A Randomized Hybrid-Effectiveness Trial Comparing Pharmacogenomics (PGx) to Standard Care: The PGx Applied to Chronic Pain Treatment in Primary Care (PGx-ACT) Trial. Clin. Transl. Sci., 18, e70154 (2025).
3. Smith, D.M. et al. CYP2D6-guided opioid therapy improves pain control in CYP2D6 intermediate and poor metabolizers: a pragmatic clinical trial. Genet. Med., 21, 1842-1850 (2019).

The comment feature is locked by administrator.