Author: Min Zhu, PhD on November 24, 2025 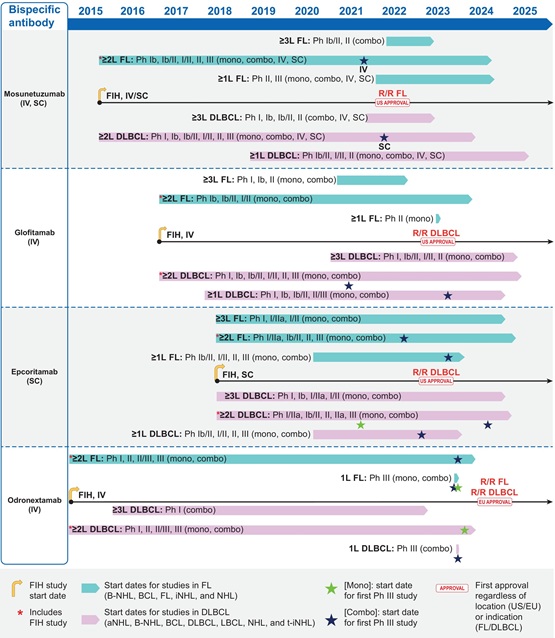
Therapeutics for B-cell non-Hodgkin lymphoma (B-NHL) have advanced over recent decades, with immunotherapies taking a central role in the evolving treatment paradigm. Bispecific antibodies (bsAbs) that engage T cells (via CD3) and malignant B cells (via CD20), including four CD20×CD3 bsAbs (mosunetuzumab, glofitamab, epcoritamab, and odronextamab), have been investigated in patients with B-NHL. This review provides a comprehensive overview of the clinical development of these four bsAbs, focusing on the unique challenges that present with this drug class, and the key strategies to address.
We describe the developmental pathways including initial in vitro evaluations for learning basic drug properties; translational assessments in preclinical animal models to understand antitumor effects as well as pharmacokinetic, pharmacodynamic, and toxicity profiles; first‑in‑human dose projection; and determination of treatment regimens for Phase 2/3 studies.
Data obtained from first-in-human studies provided the most relevant information for regimen selection and optimization of Phase 2/3 studies. Application of various modelling and simulation approaches for each bsAb was critical to identify dosing regimens with balanced safety and efficacy profiles. These models accounted for cytokine release, dynamics of immune cells, and interactions of bsAbs with T cells and B cells. Importantly, optimization of step-up dosing is essential for mitigating the risk of cytokine release syndrome. With optimized treatment regimens for each bsAb, multiple focused Phase 1/2 trials were conducted in parallel to evaluate drug effects in early lines of therapy and in multiple treatment settings, either as monotherapy or in combination with existing therapies. These approaches appeared effective in obtaining the required data to initiate confirmatory Phase 3 studies. Additionally, our detailed review highlights variations in clinical trial strategy between bsAbs, especially in timing, study design, and sample size.
The developmental approaches employed for mosunetuzumab, glofitamab, epcoritamab, and odronextamab described here provide valuable information that could help support efficient development of similar classes of bsAbs in the future.

The comment feature is locked by administrator.