Author: Ping Zhao, PhD and Piet H. van der Graaf, PhD, PharmD on April 19, 2018 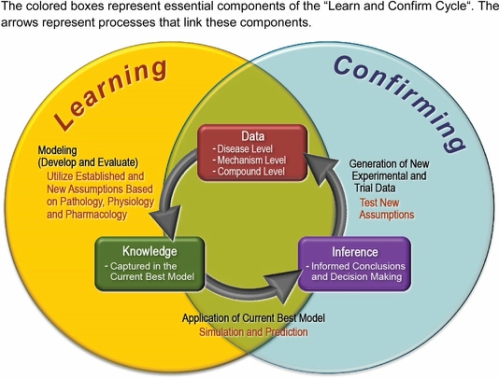
On April 17, 2018, the US FDA announced the Model-Informed Drug Development Pilot Program. The program fulfills one of the agency’s performance goals under its 6th iteration of the Prescription Drug User Fee Act (DUFA VI, 2018-2022), which recognizes the importance of model-informed drug development (MiDD). Under the Program, sponsors can request meetings with the FDA to specifically discuss the use of modeling and simulations (M&S) as early as pre-IND (Investigational New Drug Application).
The announcement came six days after the agency published the new guidance “E11(R1) Addendum: Clinical Investigation of Medicinal Products in the Pediatric Population,” which introduces M&S in pediatric drug development. The closeness of these two events provides unique opportunities for drug sponsors who are interested in streamlining pediatric drug development to request meetings with the agency on the use of M&S.
One feature of the MiDD pilot program is that consortia or software/device developers can join the meetings between the FDA and the sponsor. This feature can be relevant to M&S for pediatric assessment. According to the new guidance, models for pediatric assessment should consider findings of the drug itself and drug-independent information, such as disease and developmental physiology. Input from consortia or developers that are experienced in developing such systems models can facilitate the discussion.
The systems models are more crucial for the development of drugs to treat infectious diseases in young children in developing countries. In these countries, infections are the top reasons for the death of children aged 1-59 months. Patient factors specific to these young children can significantly alter drug behavior (e.g., compromised immune function as a result of malnutrition and/or HIV co-infection, co-infection of other pathogens), and cannot be readily evaluated in trials conducted in adults and older children. M&S thus presents a viable means by incorporating these factors to support pediatric dose selection.
In conclusion, the MiDD pilot program allows sponsors to have early discussions with the FDA on the use of complex systems models in pediatric drug development.
Disclaimer: The views do not reflect those of the Bill & Melinda Gates Foundation.
Image by Marshall, et al. CPT Pharmacometrics Syst. Pharmacol., doi: 10.1002/psp4.12049, is licensed under CC BY-NC 4.0. ©2016 The authors.

The comment feature is locked by administrator.