Author: Gelareh Abulwerdi, PhD on June 25, 2024 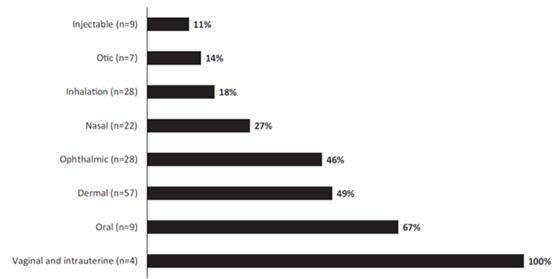
Many drugs that are currently approved in children are initially developed in adults. Adult data is used to inform dosing for pediatric studies through leveraging pharmacokinetic (PK) data such as exposure matching to adults. However, locally acting drugs (LADs) have limited systemic exposure and the use of such approaches in LAD product development is limited. In this paper, we surveyed a large database of LADs that were assessed in pediatric patients to understand their drug development programs with a focus on comparing dosing information between adults and pediatrics.
Our data suggests similarity in dosing approaches for LADs between adults and pediatric patients despite challenges such as limitation in utilization of approaches such as exposure matching. Furthermore, there was some use of extrapolation of efficacy approach from adults or older pediatric population to younger pediatric population in LAD submissions with majority in vaginal and intrauterine submissions, oral, dermal, and ophthalmic submissions. Notably, the subset of LADs that are drug-device combination products such as nasal and inhalation and injectable products reported the biggest size of pediatric clinical trials, minimal usage of extrapolation of efficacy approach, and they assessed more than one dose and/or dosing regimen in their pediatric development programs. This highlights the need to further study these group of drugs to streamline their pediatric drug development programs.

The comment feature is locked by administrator.