Author: Ki Young Huh, MD, PhD on May 06, 2025 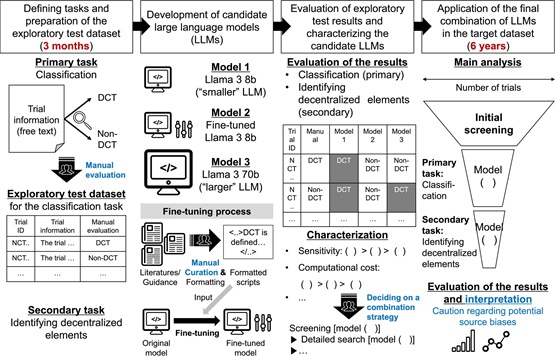
There is growing interest in using large language models (LLMs) to support clinical research. In particular, research on clinical trial registries has traditionally required considerable manual curation of data, which has hindered timely and up-to-date investigations on various topics. We hypothesize that LLMs could help make this type of research more efficient.
We focused on clinical trials involving decentralized elements (DCTs) as an ideal area for applying LLMs. DCTs are defined as trials that involve trial-related activities outside of conventional trial sites. These decentralized elements can take many forms, from simply electronic diaries to the use of local healthcare facilities and direct-to-patient shipments. From an industry perspective, DCTs have increased sharply, especially with the COVID-19 pandemic. Unfortunately, there have been few studies on clinical trial registries that provide concrete evidence of an increase.
The primary reason for the lack of research in this area is the highly heterogeneous way in which DCTs are described. First and foremost, the term "DCT" has only recently been established, with other terms (e.g., virtual trial, mobile trial) also being used. Additionally, not all trials explicitly mention "DCTs," even if they widely adopt decentralized elements. As a result, studying the trend of DCTs is particularly challenging.
We propose a practical workflow for studying clinical trials using open-source LLMs. We selected one of the available LLMs and fine-tuned the model with expert-curated data. We then applied the model to trial information from the ClinicalTrials.gov registry to classify and, where possible, extract decentralized elements through prompt engineering. Our findings suggest that while the performance of LLMs requires further improvement, these models have the potential to support research on clinical trial registries. We anticipate that further research into LLMs could enable up-to-date studies and curation of data from large databases including, but not limited to, clinical trial registries.

The comment feature is locked by administrator.