Author: Cindy H. T. Yeung, MSc, PhD; Deanna C. Sekulich, BS; Tamorah Lewis, MD, PhD; Jeff Reese, MD; Anil Maharaj, BSc (Pharm), PhD on January 27, 2026 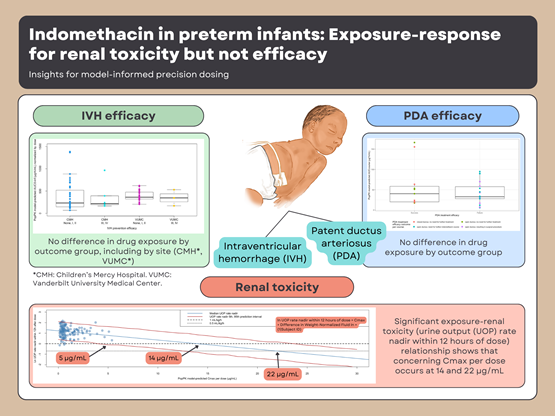
Indomethacin is the 10th most commonly used medication in Neonatal Intensive Care Units (NICU) for two conditions: intraventricular hemorrhage (IVH) and patent ductus arteriosus (PDA); however, current dosing has not been evidence-based. Standard weight-based dosing often leads to unpredictable clinical efficacy and toxicity. Studies in the 1980s attempted to define the relationship between indomethacin exposure and response to have an improved understanding of dosing strategies but were limited by sample size, focused solely on PDA patients, and/or did not use robust exposure estimates (such as AUC).
Based on a cohort of 83 neonates across two institutions, a modified indomethacin population pharmacokinetic model was used to produce indomethacin exposure estimates. We evaluated the relationship between exposure and both efficacy (IVH severity and PDA success) and renal toxicity (very low urine output rates).
We found:
- Despite the widespread use of the current dosing regimen (0.1 mg/kg every 24 hours x3 for IVH; and 0.1 mg/kg every 12 hours x3 per course of indomethacin for PDA, with a maximum of three courses), there was no significant relationship between indomethacin exposure and efficacy.
- A simplified single dose regimen of 0.2 mg/kg resulted in similar exposure-response profile in IVH prevention as the typical dosing scheme in #1
- Indomethacin significantly reduced IVH severity as compared to no indomethacin
- A significant relationship between indomethacin exposure and renal toxicity (urine output decline) with potential renal toxicity occurring at indomethacin concentrations as low as 5 µg/mL
Our work defines an upper limit for maximum concentrations upon which renal toxicity can occur and helps inform dose increases from a safety perspective. Future studies should focus on defining a therapeutic index considering both safety and efficacy to better inform NICU dosing of indomethacin.

The comment feature is locked by administrator.