Author: Ping Zhao, PhD and Vikram Sinha, PhD on October 24, 2018 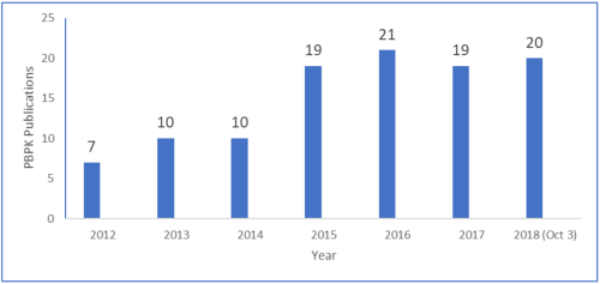
On September 3, 2018, the US Food and Drug Administration (FDA) finalized its first PBPK guidance: Physiologically Based Pharmacokinetic Analyses — Format and Content following the draft guidance in December 2016. The guidance followed key workshops hosted by the FDA and European Medicines Agency (EMA) in 2014. This speedy development of PBPK policy represents FDA’s recognition of the utility of PBPK, amongst other model-informed drug development methods, in drug development and regulations.
Responding to significant variation of PBPK submissions, the guidance provides direction towards the standardization of the content and format of these reports to facilitate review and decision making. The content of the final guidance is generally consistent with that in the draft, with noticeable edits intended to address public comments and to enhance clarity. Salient features of the guidance are:
- It articulates that analyses and discussion should be in the context of intended use.
- Under “Software” section, additional information may be needed to ensure that the FDA has sufficient working knowledge of the program being used. This is an important message for a sponsor to not only be conscious about limited resources at FDA, but also engage the agency early if they anticipate reviewers are unfamiliar with the software or platform the sponsor employs.
- The guidance includes new details on submitting electronic model files.
Other updates include flexibility in recommendations, the need to describe selection of parameter values and, the inclusion of dosing regimen information under simulation design.
Under the umbrella of systems pharmacology, PBPK continues to be used, as evident from over 100 publications in CPT: Pharmacometrics & Systems Pharmacology over a 6-year period (Figure). The application has been constant since the FDA workshop in 2014.

The comment feature is locked by administrator.